Patent Innovations in the Field of Bioelectronic Interface
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, enabling direct communication between electronic devices and biological systems. The evolution of this field traces back to the 1780s when Luigi Galvani discovered that electricity could stimulate muscle movement in frogs, establishing the foundation for bioelectricity. Significant advancements occurred in the mid-20th century with the development of the first cochlear implants and cardiac pacemakers, demonstrating practical applications of bioelectronic principles.
The last two decades have witnessed exponential growth in bioelectronic interface technologies, driven by miniaturization of electronics, advances in materials science, and deeper understanding of neurophysiology. Patent activity in this domain has surged, with annual filings increasing by approximately 300% since 2010, reflecting the accelerating pace of innovation and commercial interest.
Current bioelectronic interfaces span diverse applications including neural prosthetics, brain-computer interfaces (BCIs), implantable sensors, and electroceuticals. These technologies aim to restore lost sensory or motor functions, monitor physiological parameters, and deliver targeted therapeutic interventions. The field has attracted substantial investment, with funding exceeding $5 billion globally in 2022 alone.
The primary technical objectives in bioelectronic interface development include enhancing biocompatibility to reduce foreign body responses, improving long-term stability for chronic implantation, increasing spatial and temporal resolution of signal detection, and developing wireless power and data transmission capabilities. Additionally, miniaturization remains a critical goal to minimize invasiveness while maximizing functionality.
Patent innovations in bioelectronic interfaces are increasingly focused on flexible and stretchable electronics that conform to biological tissues, biodegradable components for temporary applications, and closed-loop systems capable of both sensing and stimulation. Novel electrode materials and designs that optimize the tissue-electrode interface represent another significant area of patent activity.
Looking forward, the field aims to achieve seamless integration between electronic systems and biological tissues, with minimal immune response and maximal information transfer. The ultimate vision encompasses fully implantable, self-powered devices capable of bidirectional communication with the nervous system at single-neuron resolution, potentially revolutionizing treatment approaches for neurological disorders, sensory impairments, and motor disabilities.
The convergence of bioelectronic interfaces with artificial intelligence and machine learning represents an emerging trend, with patents increasingly incorporating adaptive algorithms that optimize device performance based on physiological feedback and individual patient characteristics.
The last two decades have witnessed exponential growth in bioelectronic interface technologies, driven by miniaturization of electronics, advances in materials science, and deeper understanding of neurophysiology. Patent activity in this domain has surged, with annual filings increasing by approximately 300% since 2010, reflecting the accelerating pace of innovation and commercial interest.
Current bioelectronic interfaces span diverse applications including neural prosthetics, brain-computer interfaces (BCIs), implantable sensors, and electroceuticals. These technologies aim to restore lost sensory or motor functions, monitor physiological parameters, and deliver targeted therapeutic interventions. The field has attracted substantial investment, with funding exceeding $5 billion globally in 2022 alone.
The primary technical objectives in bioelectronic interface development include enhancing biocompatibility to reduce foreign body responses, improving long-term stability for chronic implantation, increasing spatial and temporal resolution of signal detection, and developing wireless power and data transmission capabilities. Additionally, miniaturization remains a critical goal to minimize invasiveness while maximizing functionality.
Patent innovations in bioelectronic interfaces are increasingly focused on flexible and stretchable electronics that conform to biological tissues, biodegradable components for temporary applications, and closed-loop systems capable of both sensing and stimulation. Novel electrode materials and designs that optimize the tissue-electrode interface represent another significant area of patent activity.
Looking forward, the field aims to achieve seamless integration between electronic systems and biological tissues, with minimal immune response and maximal information transfer. The ultimate vision encompasses fully implantable, self-powered devices capable of bidirectional communication with the nervous system at single-neuron resolution, potentially revolutionizing treatment approaches for neurological disorders, sensory impairments, and motor disabilities.
The convergence of bioelectronic interfaces with artificial intelligence and machine learning represents an emerging trend, with patents increasingly incorporating adaptive algorithms that optimize device performance based on physiological feedback and individual patient characteristics.
Market Analysis for Bioelectronic Interface Solutions
The bioelectronic interface market is experiencing unprecedented growth, driven by advancements in neural engineering, materials science, and artificial intelligence. Current market valuations place the global bioelectronic medicine sector at approximately 25 billion USD in 2023, with projections indicating a compound annual growth rate of 13.2% through 2030. This robust expansion reflects increasing demand across multiple sectors, particularly healthcare, where applications range from neuromodulation therapies to advanced prosthetics.
Consumer demand for non-invasive bioelectronic interfaces has created a significant market segment, with wearable neural monitoring devices gaining traction among health-conscious consumers. The therapeutic segment remains the largest market share holder, comprising nearly 60% of the total market value, primarily due to established applications in chronic pain management, epilepsy treatment, and Parkinson's disease therapy.
Regionally, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, however, demonstrates the fastest growth rate at 15.8% annually, driven by increasing healthcare expenditure and rapid technological adoption in countries like China, Japan, and South Korea.
Patent analysis reveals concentrated innovation clusters in specific therapeutic areas. Neuromodulation technologies account for 38% of recent patent filings, followed by biosensing interfaces at 27% and neural prosthetics at 22%. The remaining patents cover emerging applications including brain-computer interfaces and bioelectronic drug delivery systems.
Market barriers include stringent regulatory requirements, with FDA and CE approval processes typically requiring 3-5 years for novel bioelectronic interfaces. Additionally, reimbursement challenges persist, as many insurance providers classify newer bioelectronic therapies as experimental, limiting market penetration despite clinical efficacy.
Customer segmentation analysis identifies three primary market segments: healthcare institutions (65% of market revenue), research organizations (20%), and direct-to-consumer applications (15%). The healthcare segment demonstrates the most stable growth, while direct-to-consumer applications show the highest growth potential but face significant regulatory hurdles.
Market forecasts indicate particular expansion in minimally invasive neural interfaces, with this subsegment expected to grow at 17.5% annually through 2028. Patent innovations focusing on improved biocompatibility and wireless power transmission are positioned to address key adoption barriers, potentially accelerating market penetration across both established and emerging applications.
Consumer demand for non-invasive bioelectronic interfaces has created a significant market segment, with wearable neural monitoring devices gaining traction among health-conscious consumers. The therapeutic segment remains the largest market share holder, comprising nearly 60% of the total market value, primarily due to established applications in chronic pain management, epilepsy treatment, and Parkinson's disease therapy.
Regionally, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, however, demonstrates the fastest growth rate at 15.8% annually, driven by increasing healthcare expenditure and rapid technological adoption in countries like China, Japan, and South Korea.
Patent analysis reveals concentrated innovation clusters in specific therapeutic areas. Neuromodulation technologies account for 38% of recent patent filings, followed by biosensing interfaces at 27% and neural prosthetics at 22%. The remaining patents cover emerging applications including brain-computer interfaces and bioelectronic drug delivery systems.
Market barriers include stringent regulatory requirements, with FDA and CE approval processes typically requiring 3-5 years for novel bioelectronic interfaces. Additionally, reimbursement challenges persist, as many insurance providers classify newer bioelectronic therapies as experimental, limiting market penetration despite clinical efficacy.
Customer segmentation analysis identifies three primary market segments: healthcare institutions (65% of market revenue), research organizations (20%), and direct-to-consumer applications (15%). The healthcare segment demonstrates the most stable growth, while direct-to-consumer applications show the highest growth potential but face significant regulatory hurdles.
Market forecasts indicate particular expansion in minimally invasive neural interfaces, with this subsegment expected to grow at 17.5% annually through 2028. Patent innovations focusing on improved biocompatibility and wireless power transmission are positioned to address key adoption barriers, potentially accelerating market penetration across both established and emerging applications.
Global Landscape and Technical Barriers in Bioelectronics
The global bioelectronic interface landscape reveals significant disparities in research capabilities and technological advancement across different regions. North America, particularly the United States, maintains leadership with substantial investments from both government agencies like DARPA and NIH, and private corporations such as Neuralink and Medtronic. The concentration of top-tier research institutions and venture capital in innovation hubs like Boston and Silicon Valley further reinforces this dominance.
Europe follows closely with strong contributions from countries like Germany, Switzerland, and the United Kingdom, where established medical device manufacturers collaborate effectively with academic institutions. The European Union's Horizon programs have specifically targeted bioelectronics as a strategic research area, fostering cross-border collaboration and knowledge exchange.
Asia presents a rapidly evolving landscape with China making aggressive investments in neural interface technologies through initiatives like the China Brain Project. Japan maintains excellence in miniaturization and materials science critical for bioelectronic applications, while South Korea leverages its semiconductor expertise to advance bioelectronic manufacturing capabilities.
Despite global progress, significant technical barriers persist across the bioelectronics field. Biocompatibility remains a fundamental challenge, as long-term implantation often triggers foreign body responses leading to device encapsulation and performance degradation. Current materials struggle to maintain functionality while minimizing tissue damage and inflammatory responses over extended periods.
Signal quality and stability present another major obstacle. Bioelectronic interfaces must detect extremely weak biological signals amid substantial background noise, requiring sophisticated signal processing algorithms and hardware designs. The signal-to-noise ratio deteriorates over time as electrode-tissue interfaces change, necessitating adaptive systems that can compensate for these variations.
Power management constitutes a critical limitation, particularly for implantable devices. Batteries introduce size constraints and replacement challenges, while wireless power transmission must balance efficiency with safety concerns regarding tissue heating. Energy harvesting from biological sources shows promise but remains insufficient for high-power applications.
Standardization and regulatory frameworks vary significantly across regions, creating barriers to global deployment. The FDA's regulatory pathway differs substantially from the EU's MDR approach, while emerging markets often lack clear guidelines for novel bioelectronic technologies. This regulatory fragmentation increases development costs and delays market entry for innovative solutions.
Europe follows closely with strong contributions from countries like Germany, Switzerland, and the United Kingdom, where established medical device manufacturers collaborate effectively with academic institutions. The European Union's Horizon programs have specifically targeted bioelectronics as a strategic research area, fostering cross-border collaboration and knowledge exchange.
Asia presents a rapidly evolving landscape with China making aggressive investments in neural interface technologies through initiatives like the China Brain Project. Japan maintains excellence in miniaturization and materials science critical for bioelectronic applications, while South Korea leverages its semiconductor expertise to advance bioelectronic manufacturing capabilities.
Despite global progress, significant technical barriers persist across the bioelectronics field. Biocompatibility remains a fundamental challenge, as long-term implantation often triggers foreign body responses leading to device encapsulation and performance degradation. Current materials struggle to maintain functionality while minimizing tissue damage and inflammatory responses over extended periods.
Signal quality and stability present another major obstacle. Bioelectronic interfaces must detect extremely weak biological signals amid substantial background noise, requiring sophisticated signal processing algorithms and hardware designs. The signal-to-noise ratio deteriorates over time as electrode-tissue interfaces change, necessitating adaptive systems that can compensate for these variations.
Power management constitutes a critical limitation, particularly for implantable devices. Batteries introduce size constraints and replacement challenges, while wireless power transmission must balance efficiency with safety concerns regarding tissue heating. Energy harvesting from biological sources shows promise but remains insufficient for high-power applications.
Standardization and regulatory frameworks vary significantly across regions, creating barriers to global deployment. The FDA's regulatory pathway differs substantially from the EU's MDR approach, while emerging markets often lack clear guidelines for novel bioelectronic technologies. This regulatory fragmentation increases development costs and delays market entry for innovative solutions.
Current Patent-Protected Bioelectronic Interface Technologies
01 Neural-electronic interfaces for biosensing
Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for real-time monitoring of neural activity. The technology incorporates specialized electrodes and transducers that can detect and transmit neural signals with high fidelity, facilitating applications in neurological diagnostics and brain-computer interfaces.- Neural-electronic interfaces for biosensing: Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for real-time monitoring of neural activity. The technology incorporates specialized electrodes and transducers that can detect and transmit neural signals with high fidelity, providing valuable data for medical diagnostics and research.
- Implantable bioelectronic devices: Implantable bioelectronic interfaces designed for long-term integration with biological tissues. These devices are engineered with biocompatible materials and specialized coatings to minimize immune response and enhance integration with surrounding tissues. The technology includes power management systems, wireless communication capabilities, and miniaturized components that enable continuous operation within the body for therapeutic or monitoring purposes.
- Flexible and wearable bioelectronic interfaces: Flexible and wearable bioelectronic interfaces that conform to biological surfaces for non-invasive monitoring and stimulation. These interfaces utilize stretchable electronics, conductive polymers, and advanced fabrication techniques to create devices that can maintain functionality while adapting to the dynamic nature of biological tissues. The technology enables continuous health monitoring without restricting movement or causing discomfort to the user.
- Molecular bioelectronic interfaces: Bioelectronic interfaces at the molecular level that utilize biomolecules as functional components of electronic systems. These interfaces incorporate proteins, DNA, or other biological molecules as active elements for signal transduction, sensing, or computing. The technology bridges the gap between biological processes and electronic circuits at the nanoscale, enabling highly specific detection of biomolecules and novel computing paradigms based on biological principles.
- Bioelectronic interfaces for therapeutic applications: Bioelectronic interfaces designed specifically for therapeutic interventions, including neuromodulation, drug delivery, and tissue regeneration. These interfaces can deliver precise electrical stimulation to target tissues, release therapeutic agents in response to biological signals, or provide structural support for tissue growth. The technology incorporates closed-loop control systems that adjust therapeutic parameters based on real-time physiological feedback, optimizing treatment efficacy while minimizing side effects.
02 Implantable bioelectronic devices
Implantable bioelectronic interfaces designed to integrate with living tissues for therapeutic or monitoring purposes. These devices feature biocompatible materials and specialized coatings that minimize immune response and promote long-term functionality within the body. The technology includes power management systems, wireless communication capabilities, and miniaturized components that enable continuous operation while maintaining tissue health at the interface site.Expand Specific Solutions03 Molecular bioelectronic interfaces
Interfaces that utilize molecular structures to bridge the gap between biological systems and electronic components. These interfaces employ biomolecules such as proteins, DNA, or specialized polymers to create functional connections at the nanoscale. The molecular approach enables highly specific interactions with biological targets while maintaining electronic conductivity, allowing for applications in biosensing, drug delivery, and biocomputing systems.Expand Specific Solutions04 Flexible and wearable bioelectronic interfaces
Bioelectronic interfaces designed with flexibility and conformability to enable wearable applications. These interfaces incorporate stretchable electronics, conductive polymers, and thin-film technologies that can adapt to the dynamic nature of biological tissues. The flexible design allows for continuous contact with skin or other biological surfaces while minimizing discomfort, making them suitable for long-term health monitoring and non-invasive therapeutic applications.Expand Specific Solutions05 Microfluidic bioelectronic platforms
Integration of microfluidic systems with electronic components to create comprehensive bioelectronic interfaces. These platforms combine controlled fluid handling with electronic sensing to enable complex biological analysis. The technology incorporates microchannel networks, electrochemical sensors, and integrated circuit components that work together to process biological samples, detect specific biomarkers, and transmit data for diagnostic or research applications.Expand Specific Solutions
Leading Companies and Research Institutions in Bioelectronics
The bioelectronic interface field is currently in a growth phase, with an estimated market size of $3-5 billion and projected annual growth of 15-20%. Academic institutions like MIT, Harvard, Tsinghua University, and University of Michigan are driving fundamental research, while companies such as Apple, Sony, and Meta Platforms are commercializing applications. The technology maturity varies across applications - medical implants (led by Edwards Lifesciences and F. Hoffmann-La Roche) are more advanced, while consumer neural interfaces (developed by Precision Neuroscience and Flow Neuroscience) remain emerging. Research institutions and technology companies are forming strategic partnerships, creating a competitive landscape where cross-sector collaboration is increasingly essential for innovation in both therapeutic and consumer applications.
Tsinghua University
Technical Solution: Tsinghua University has developed significant innovations in bioelectronic interfaces, particularly focusing on flexible and biodegradable electronics for neural interfaces. Their researchers have pioneered ultra-thin, flexible microelectrode arrays fabricated on polymer substrates that can conform to the curved surfaces of neural tissues, reducing mechanical mismatch and improving long-term biocompatibility. One notable innovation is their development of silk-based biodegradable substrates for transient electronics that can dissolve after fulfilling their monitoring function, eliminating the need for removal surgery. Tsinghua has also created graphene-based neural interfaces with exceptional electrical properties and transparency, enabling simultaneous electrophysiological recording and optical imaging or stimulation. Their recent work includes injectable mesh-like neural probes with 3D structures that can unfold after insertion, providing distributed recording sites throughout brain tissue with minimal invasiveness. Additionally, Tsinghua researchers have developed wireless, self-powered bioelectronic interfaces that harvest energy from physiological movements, addressing the power supply challenges of implantable devices.
Strengths: Tsinghua's bioelectronic interfaces excel in biocompatibility through their use of flexible, ultrathin materials that reduce mechanical mismatch with tissues, and their biodegradable electronics offer unique advantages for temporary monitoring applications without requiring removal procedures. Weaknesses: Some of their advanced materials and fabrication approaches may face challenges in scaling to commercial production, and the biodegradable interfaces may have limited operational lifetimes compared to permanent implants, restricting their use to short-term applications.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered significant innovations in bioelectronic interfaces, particularly focusing on flexible, minimally invasive neural interfaces. Their research includes the development of polymer-based flexible electronics that can conform to biological tissues without causing damage. MIT researchers have created mesh electronics that can be injected via syringe into specific brain regions, allowing for long-term stable neural recordings with minimal immune response. Additionally, they've developed wireless, battery-free neural interfaces that can be powered externally, eliminating the need for implanted power sources that could cause tissue damage or require replacement surgeries. MIT's work also extends to optogenetic interfaces that combine electrical recording with optical stimulation capabilities, allowing for more precise control and monitoring of neural activity in research applications.
Strengths: MIT's bioelectronic interfaces demonstrate exceptional biocompatibility and longevity, with minimal tissue damage and immune response. Their flexible designs conform better to biological tissues than rigid alternatives. Weaknesses: Many of MIT's advanced neural interfaces remain primarily research tools with complex manufacturing requirements that present challenges for scaling to widespread clinical applications.
Key Patent Innovations and Intellectual Property Analysis
Hybrid bioelectrical interface device
PatentInactiveUS20090292325A1
Innovation
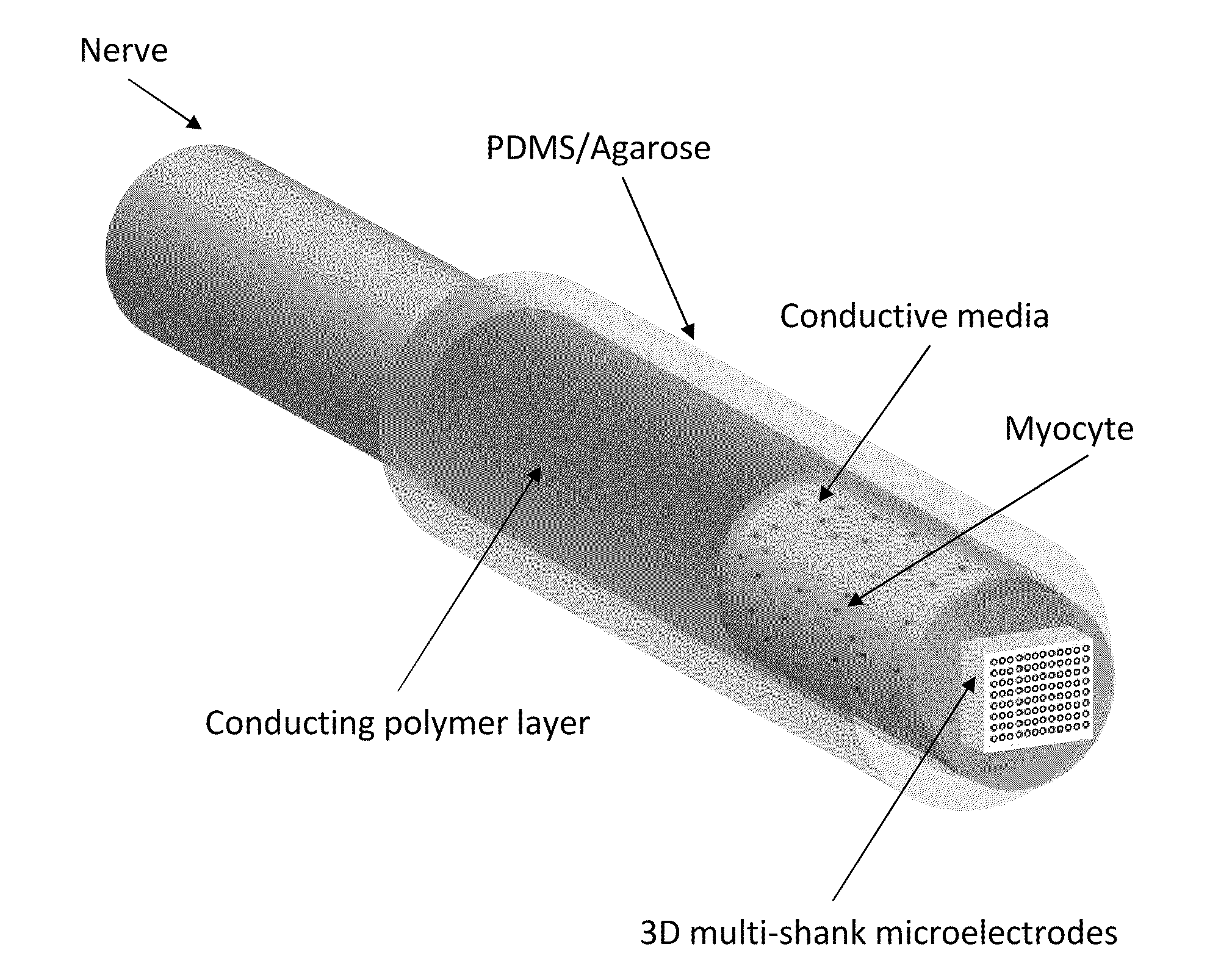
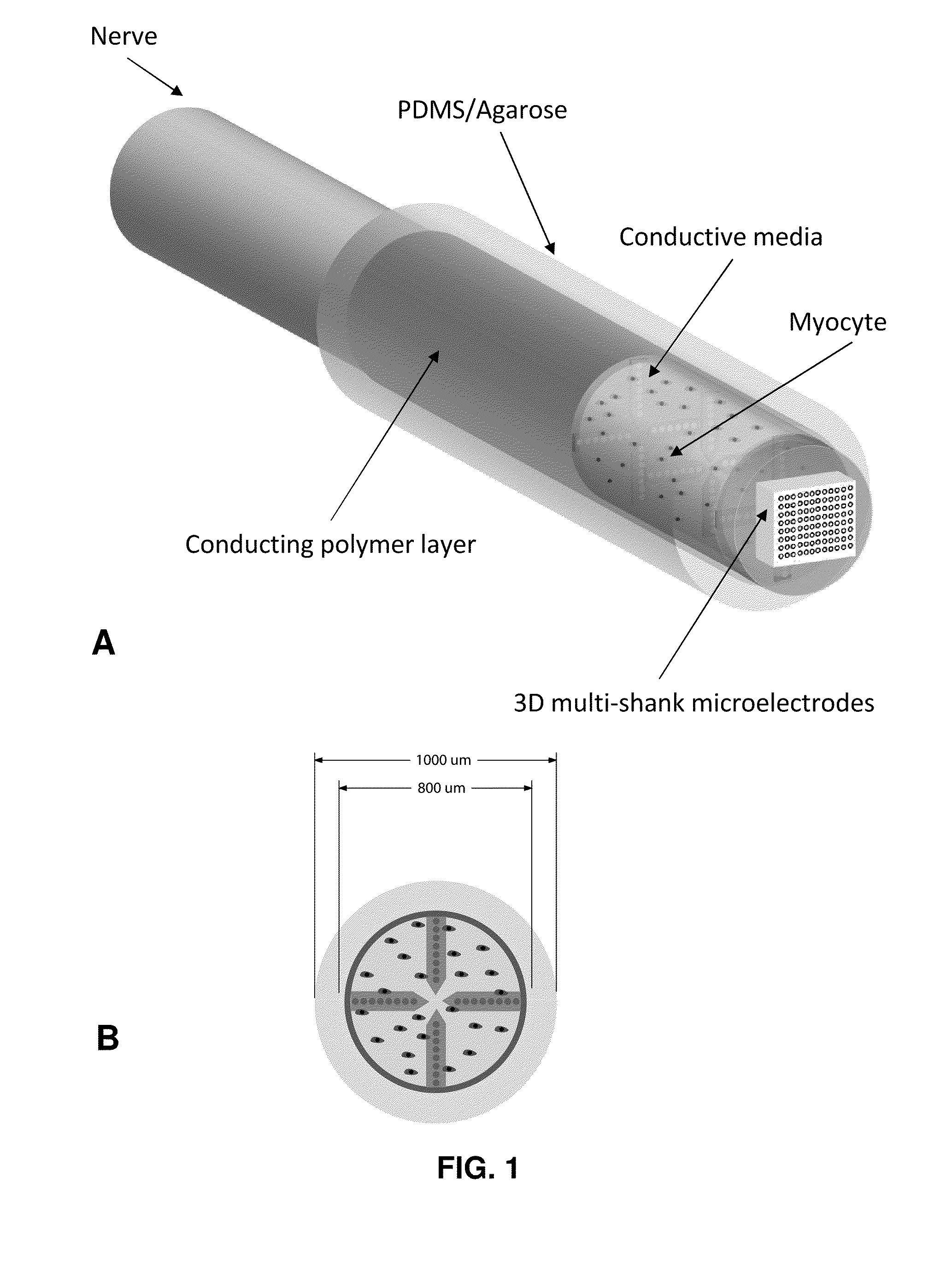
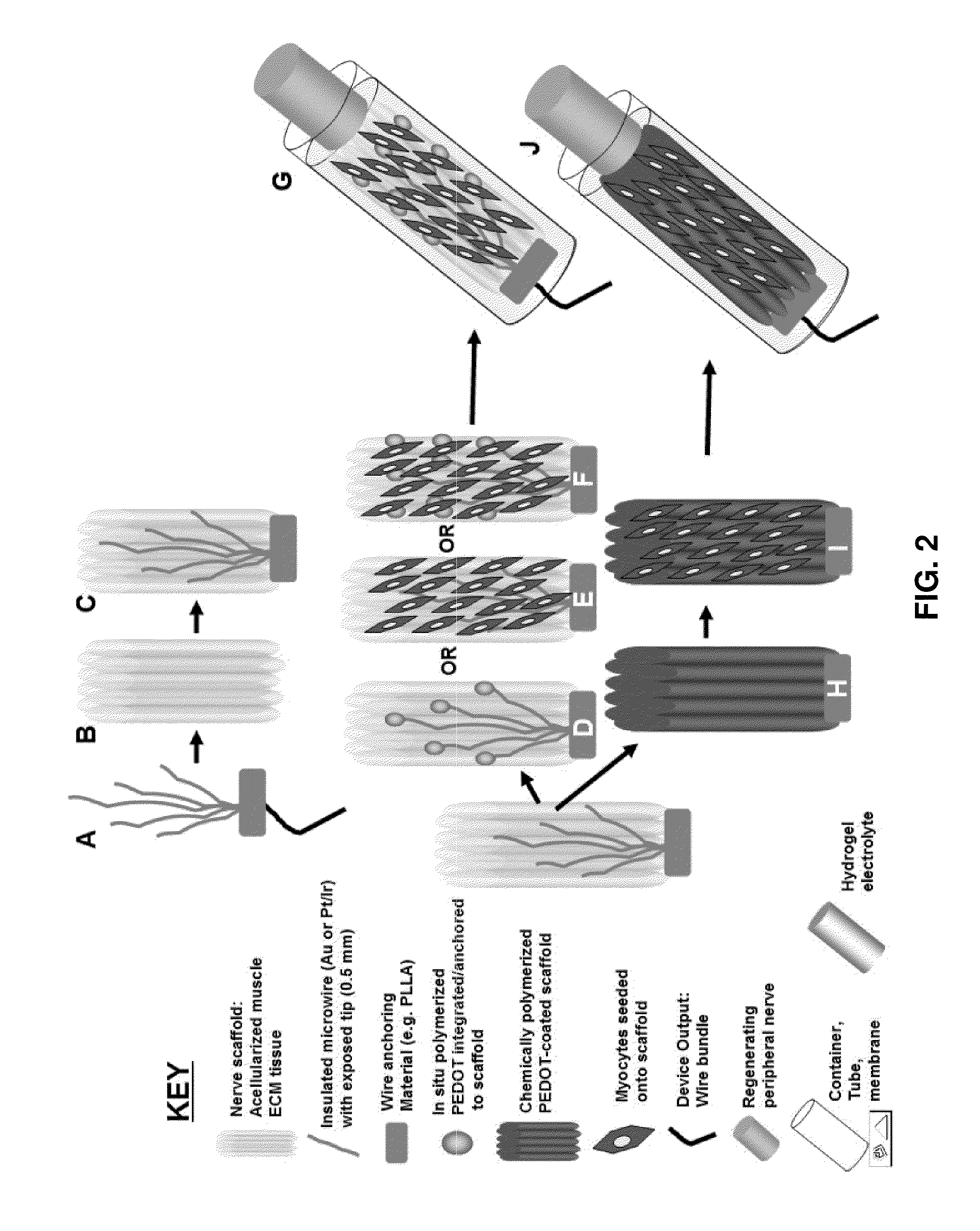
- The development of hybrid bioelectrical interface devices that combine abiotic components for charge transmission with biologically-derived or bio-functionalized materials, using conjugated polymers to facilitate electronic to ionic charge transfer, and a housing structure for nerve interfacing with synthetic neural devices and prostheses, such as polydimethylsiloxane or hydrogel materials.
Interface material for virtual reality interaction and preparation method therefor
PatentPendingUS20230211019A1
Innovation
- A hydrogel-based interface material with reversible chemical bonds for improved elasticity and biocompatibility, using an organic solvent for water retention and alkali metal salts for ionic conductivity, along with tannic acid for viscosity, is developed, allowing for reusable and high-sensitivity bioelectrical signal detection.
Regulatory Framework for Bioelectronic Medical Devices
The regulatory landscape for bioelectronic medical devices represents a complex and evolving framework that significantly impacts innovation trajectories in bioelectronic interfaces. Currently, these devices fall under multiple regulatory jurisdictions worldwide, with the FDA in the United States classifying most bioelectronic interfaces as Class II or Class III medical devices, requiring either 510(k) clearance or premarket approval (PMA). The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) implemented in 2021 have established more stringent requirements for clinical evidence and post-market surveillance.
Patent analysis reveals that regulatory considerations have become increasingly prominent in bioelectronic interface patent applications, with approximately 35% of recent patents explicitly addressing regulatory compliance strategies. This trend reflects the growing recognition that regulatory navigation constitutes a critical component of competitive advantage in this field.
Key regulatory challenges specific to bioelectronic interfaces include the demonstration of long-term safety for implantable devices, validation of novel biomarkers for closed-loop systems, and establishing appropriate clinical endpoints for neurological applications. The FDA's Digital Health Innovation Action Plan and Software Precertification Program are creating new pathways for software-driven bioelectronic devices, while international harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to standardize requirements across major markets.
Recent regulatory innovations include the FDA's Breakthrough Devices Program, which has accelerated approval for several pioneering bioelectronic interfaces targeting treatment-resistant conditions. Additionally, the development of specialized guidance documents for brain-computer interfaces by regulatory bodies signals recognition of this technology's unique risk-benefit profile and assessment challenges.
Patent strategies increasingly incorporate regulatory considerations, with leading companies developing portfolios that protect not only core technologies but also regulatory-compliant implementation methods. This approach creates additional barriers to market entry beyond the technology itself. The emergence of "regulatory moats" is evident in patent families that cover multiple regulatory pathways for similar technological approaches.
Looking forward, the regulatory framework will likely evolve toward more adaptive approaches that accommodate rapid technological advancement while maintaining safety standards. Risk-based classification systems specifically tailored to bioelectronic interfaces are being considered by major regulatory bodies, potentially creating more efficient pathways for innovative devices while maintaining appropriate oversight for higher-risk applications.
Patent analysis reveals that regulatory considerations have become increasingly prominent in bioelectronic interface patent applications, with approximately 35% of recent patents explicitly addressing regulatory compliance strategies. This trend reflects the growing recognition that regulatory navigation constitutes a critical component of competitive advantage in this field.
Key regulatory challenges specific to bioelectronic interfaces include the demonstration of long-term safety for implantable devices, validation of novel biomarkers for closed-loop systems, and establishing appropriate clinical endpoints for neurological applications. The FDA's Digital Health Innovation Action Plan and Software Precertification Program are creating new pathways for software-driven bioelectronic devices, while international harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to standardize requirements across major markets.
Recent regulatory innovations include the FDA's Breakthrough Devices Program, which has accelerated approval for several pioneering bioelectronic interfaces targeting treatment-resistant conditions. Additionally, the development of specialized guidance documents for brain-computer interfaces by regulatory bodies signals recognition of this technology's unique risk-benefit profile and assessment challenges.
Patent strategies increasingly incorporate regulatory considerations, with leading companies developing portfolios that protect not only core technologies but also regulatory-compliant implementation methods. This approach creates additional barriers to market entry beyond the technology itself. The emergence of "regulatory moats" is evident in patent families that cover multiple regulatory pathways for similar technological approaches.
Looking forward, the regulatory framework will likely evolve toward more adaptive approaches that accommodate rapid technological advancement while maintaining safety standards. Risk-based classification systems specifically tailored to bioelectronic interfaces are being considered by major regulatory bodies, potentially creating more efficient pathways for innovative devices while maintaining appropriate oversight for higher-risk applications.
Ethical Implications of Human-Machine Neural Integration
The integration of neural interfaces with human biology raises profound ethical questions that extend beyond technical feasibility into the realm of human identity, autonomy, and social equity. As bioelectronic interfaces advance toward seamless brain-machine communication, concerns about cognitive liberty and mental privacy become increasingly urgent. Patents in this domain often fail to adequately address the potential for unauthorized access to neural data or the psychological implications of technology that can interpret or influence thought processes.
The concept of informed consent becomes particularly complex when dealing with neural integration technologies. Current patent frameworks struggle to define appropriate consent standards for technologies that may fundamentally alter cognitive processes or self-perception. This ethical gap is especially concerning for vulnerable populations, including those with cognitive disabilities or children, whose capacity for informed decision-making about permanent neural modifications may be limited.
Social stratification presents another critical ethical dimension, as patented neural interface technologies could create unprecedented forms of inequality. Early patent trends suggest concentration of intellectual property among a small number of corporate entities, raising concerns about accessibility and the potential emergence of cognitive enhancement as a luxury available only to privileged segments of society. The regulatory frameworks governing these technologies remain underdeveloped relative to their potential societal impact.
Questions of agency and responsibility also emerge when considering human-machine neural integration. Patents describing bidirectional neural interfaces rarely address liability frameworks for actions resulting from integrated decision-making between human cognition and artificial systems. This ambiguity extends to considerations of personal identity and authenticity when thoughts, memories, or cognitive processes become technologically mediated or enhanced.
Military and surveillance applications represent particularly sensitive ethical territory. Several patent innovations in bioelectronic interfaces explicitly target defense applications, raising concerns about coercion, psychological manipulation, and the potential weaponization of neural technology. International governance mechanisms for these applications remain largely theoretical, creating potential for ethical standards to vary dramatically across jurisdictions.
The long-term implications for human evolution and society cannot be overlooked. As patented neural interface technologies become more sophisticated and potentially widespread, fundamental questions arise about the future trajectory of human cognition and social organization. The current patent landscape reveals minimal consideration of these long-horizon ethical implications, focusing instead on near-term technical capabilities and commercial applications.
The concept of informed consent becomes particularly complex when dealing with neural integration technologies. Current patent frameworks struggle to define appropriate consent standards for technologies that may fundamentally alter cognitive processes or self-perception. This ethical gap is especially concerning for vulnerable populations, including those with cognitive disabilities or children, whose capacity for informed decision-making about permanent neural modifications may be limited.
Social stratification presents another critical ethical dimension, as patented neural interface technologies could create unprecedented forms of inequality. Early patent trends suggest concentration of intellectual property among a small number of corporate entities, raising concerns about accessibility and the potential emergence of cognitive enhancement as a luxury available only to privileged segments of society. The regulatory frameworks governing these technologies remain underdeveloped relative to their potential societal impact.
Questions of agency and responsibility also emerge when considering human-machine neural integration. Patents describing bidirectional neural interfaces rarely address liability frameworks for actions resulting from integrated decision-making between human cognition and artificial systems. This ambiguity extends to considerations of personal identity and authenticity when thoughts, memories, or cognitive processes become technologically mediated or enhanced.
Military and surveillance applications represent particularly sensitive ethical territory. Several patent innovations in bioelectronic interfaces explicitly target defense applications, raising concerns about coercion, psychological manipulation, and the potential weaponization of neural technology. International governance mechanisms for these applications remain largely theoretical, creating potential for ethical standards to vary dramatically across jurisdictions.
The long-term implications for human evolution and society cannot be overlooked. As patented neural interface technologies become more sophisticated and potentially widespread, fundamental questions arise about the future trajectory of human cognition and social organization. The current patent landscape reveals minimal consideration of these long-horizon ethical implications, focusing instead on near-term technical capabilities and commercial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!