Research on Bioelectronic Interface Efficacy in Chronic Disease Management
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces represent a revolutionary convergence of electronics and biology, evolving significantly over the past three decades. Initially emerging in the 1990s as rudimentary neural implants, these technologies have progressed from simple stimulation devices to sophisticated bidirectional communication systems capable of both sensing biological signals and delivering precise therapeutic interventions. The miniaturization of electronic components, advancements in biocompatible materials, and breakthroughs in wireless power transmission have collectively accelerated this evolution, enabling increasingly less invasive and more effective interfaces.
The trajectory of bioelectronic interfaces has been marked by several pivotal developments. The early 2000s witnessed the introduction of deep brain stimulation systems for Parkinson's disease management, demonstrating the potential of electronic intervention in chronic conditions. By the 2010s, closed-loop systems emerged, capable of monitoring physiological parameters and automatically adjusting stimulation parameters. Recent years have seen the development of flexible, ultrathin electronics that conform to biological tissues, minimizing foreign body responses and extending device longevity.
Current bioelectronic interfaces encompass various modalities, including implantable devices, transcutaneous stimulators, and wearable systems. Each approach offers distinct advantages in terms of invasiveness, precision, and accessibility, catering to different chronic disease management needs. The integration of artificial intelligence algorithms has further enhanced these systems, enabling personalized treatment protocols that adapt to individual patient responses and disease progression patterns.
The primary objective of bioelectronic interface research in chronic disease management is to develop minimally invasive, long-lasting solutions that can modulate physiological processes with pharmaceutical-like precision but without the systemic side effects of traditional medications. Specific goals include creating interfaces that can effectively manage conditions such as rheumatoid arthritis, inflammatory bowel disease, type 2 diabetes, and treatment-resistant hypertension through targeted neuromodulation of relevant neural circuits.
Additional technical objectives include improving electrode biocompatibility to reduce tissue inflammation and fibrosis, extending battery life or developing efficient wireless power transfer mechanisms, enhancing signal processing algorithms for more accurate interpretation of biological signals, and developing adaptive stimulation protocols that respond to changing disease states. The ultimate aim is to establish bioelectronic medicine as a mainstream therapeutic approach that complements or potentially replaces conventional pharmacological interventions for chronic diseases.
The field is now moving toward increasingly personalized, closed-loop systems that continuously monitor disease biomarkers and automatically adjust therapeutic parameters. This evolution represents a paradigm shift from reactive to preventative medicine, where bioelectronic interfaces may eventually predict and preemptively address disease exacerbations before clinical symptoms manifest.
The trajectory of bioelectronic interfaces has been marked by several pivotal developments. The early 2000s witnessed the introduction of deep brain stimulation systems for Parkinson's disease management, demonstrating the potential of electronic intervention in chronic conditions. By the 2010s, closed-loop systems emerged, capable of monitoring physiological parameters and automatically adjusting stimulation parameters. Recent years have seen the development of flexible, ultrathin electronics that conform to biological tissues, minimizing foreign body responses and extending device longevity.
Current bioelectronic interfaces encompass various modalities, including implantable devices, transcutaneous stimulators, and wearable systems. Each approach offers distinct advantages in terms of invasiveness, precision, and accessibility, catering to different chronic disease management needs. The integration of artificial intelligence algorithms has further enhanced these systems, enabling personalized treatment protocols that adapt to individual patient responses and disease progression patterns.
The primary objective of bioelectronic interface research in chronic disease management is to develop minimally invasive, long-lasting solutions that can modulate physiological processes with pharmaceutical-like precision but without the systemic side effects of traditional medications. Specific goals include creating interfaces that can effectively manage conditions such as rheumatoid arthritis, inflammatory bowel disease, type 2 diabetes, and treatment-resistant hypertension through targeted neuromodulation of relevant neural circuits.
Additional technical objectives include improving electrode biocompatibility to reduce tissue inflammation and fibrosis, extending battery life or developing efficient wireless power transfer mechanisms, enhancing signal processing algorithms for more accurate interpretation of biological signals, and developing adaptive stimulation protocols that respond to changing disease states. The ultimate aim is to establish bioelectronic medicine as a mainstream therapeutic approach that complements or potentially replaces conventional pharmacological interventions for chronic diseases.
The field is now moving toward increasingly personalized, closed-loop systems that continuously monitor disease biomarkers and automatically adjust therapeutic parameters. This evolution represents a paradigm shift from reactive to preventative medicine, where bioelectronic interfaces may eventually predict and preemptively address disease exacerbations before clinical symptoms manifest.
Market Analysis for Chronic Disease Management Solutions
The chronic disease management market is experiencing significant growth, driven by the increasing prevalence of conditions such as diabetes, cardiovascular diseases, respiratory disorders, and neurological conditions. Currently valued at approximately $116 billion globally, this market is projected to reach $198 billion by 2027, representing a compound annual growth rate of 11.2%. This expansion is primarily fueled by aging populations, sedentary lifestyles, and increasing healthcare costs associated with long-term disease management.
North America dominates the chronic disease management market, accounting for nearly 40% of global revenue, followed by Europe and Asia-Pacific regions. The Asia-Pacific market, particularly China and India, is witnessing the fastest growth due to improving healthcare infrastructure, rising healthcare expenditure, and increasing awareness about disease management solutions.
The market segmentation reveals distinct categories: remote patient monitoring devices constitute about 35% of the market, medication management systems represent 25%, while telehealth services and analytics platforms each account for approximately 20%. Bioelectronic interfaces, though currently a smaller segment at around 10%, are projected to grow at the highest rate of 17.3% annually through 2027.
Consumer demand patterns indicate a strong preference for non-invasive, user-friendly solutions that integrate seamlessly with daily routines. Approximately 68% of patients with chronic conditions express interest in home-based monitoring systems, while 72% of healthcare providers acknowledge the clinical benefits of continuous patient monitoring. The willingness to adopt bioelectronic interfaces varies significantly by demographic: younger patients (18-45) show 62% acceptance rates compared to 41% among older demographics (65+).
Healthcare payers, including insurance companies and government programs, are increasingly recognizing the cost-effectiveness of preventive and continuous management approaches. Studies indicate that effective chronic disease management solutions can reduce hospital readmissions by up to 30% and emergency department visits by 25%, translating to substantial cost savings.
Regulatory environments are evolving to accommodate innovative technologies, with the FDA and European regulatory bodies establishing specialized pathways for digital health and bioelectronic solutions. Reimbursement policies are gradually adapting, with approximately 65% of developed markets now offering some form of coverage for remote monitoring technologies.
The competitive landscape features traditional medical device manufacturers expanding into digital solutions, pharmaceutical companies developing companion diagnostics and monitoring tools, and technology giants entering the healthcare space with consumer-oriented products. Strategic partnerships between technology providers and healthcare systems represent a growing trend, with over 200 significant collaborations announced in the past two years.
North America dominates the chronic disease management market, accounting for nearly 40% of global revenue, followed by Europe and Asia-Pacific regions. The Asia-Pacific market, particularly China and India, is witnessing the fastest growth due to improving healthcare infrastructure, rising healthcare expenditure, and increasing awareness about disease management solutions.
The market segmentation reveals distinct categories: remote patient monitoring devices constitute about 35% of the market, medication management systems represent 25%, while telehealth services and analytics platforms each account for approximately 20%. Bioelectronic interfaces, though currently a smaller segment at around 10%, are projected to grow at the highest rate of 17.3% annually through 2027.
Consumer demand patterns indicate a strong preference for non-invasive, user-friendly solutions that integrate seamlessly with daily routines. Approximately 68% of patients with chronic conditions express interest in home-based monitoring systems, while 72% of healthcare providers acknowledge the clinical benefits of continuous patient monitoring. The willingness to adopt bioelectronic interfaces varies significantly by demographic: younger patients (18-45) show 62% acceptance rates compared to 41% among older demographics (65+).
Healthcare payers, including insurance companies and government programs, are increasingly recognizing the cost-effectiveness of preventive and continuous management approaches. Studies indicate that effective chronic disease management solutions can reduce hospital readmissions by up to 30% and emergency department visits by 25%, translating to substantial cost savings.
Regulatory environments are evolving to accommodate innovative technologies, with the FDA and European regulatory bodies establishing specialized pathways for digital health and bioelectronic solutions. Reimbursement policies are gradually adapting, with approximately 65% of developed markets now offering some form of coverage for remote monitoring technologies.
The competitive landscape features traditional medical device manufacturers expanding into digital solutions, pharmaceutical companies developing companion diagnostics and monitoring tools, and technology giants entering the healthcare space with consumer-oriented products. Strategic partnerships between technology providers and healthcare systems represent a growing trend, with over 200 significant collaborations announced in the past two years.
Current Bioelectronic Interface Technologies and Barriers
Bioelectronic interfaces represent a rapidly evolving field at the intersection of electronics and biology, offering promising solutions for chronic disease management. Currently, these interfaces primarily consist of implantable devices, wearable sensors, and non-invasive stimulation technologies. Implantable devices such as deep brain stimulators for Parkinson's disease and vagus nerve stimulators for epilepsy have demonstrated clinical efficacy, while continuous glucose monitors have revolutionized diabetes management by providing real-time data feedback.
Wearable bioelectronic interfaces have gained significant traction due to their non-invasive nature and user accessibility. Advanced ECG patches, smart textiles with embedded sensors, and optical sensors for continuous blood pressure monitoring represent the current state-of-the-art in this category. These technologies enable longitudinal data collection in real-world settings, providing insights into disease progression patterns that were previously unattainable in clinical environments.
Despite these advancements, several critical barriers impede widespread adoption and optimal efficacy of bioelectronic interfaces in chronic disease management. Biocompatibility remains a significant challenge, particularly for long-term implantable devices, as foreign body responses can lead to inflammation, fibrosis, and eventual device failure. The development of novel biomaterials and surface modifications has shown promise in mitigating these issues, but complete biocompatibility remains elusive.
Power management presents another substantial hurdle, especially for implantable devices where battery replacement requires invasive procedures. Current solutions include wireless power transfer, energy harvesting from biological sources, and ultra-low-power circuit designs, though each approach has inherent limitations in reliability, efficiency, or implementation complexity.
Data quality and signal fidelity pose persistent challenges, particularly in ambulatory settings where motion artifacts and environmental interference can compromise measurement accuracy. Advanced signal processing algorithms and multi-sensor fusion techniques have improved reliability, but achieving clinical-grade measurements outside controlled environments remains difficult.
Regulatory pathways for bioelectronic interfaces are complex and often lengthy, with varying requirements across different regions. This regulatory landscape significantly impacts development timelines and commercialization strategies, particularly for novel technologies without predicate devices.
Integration with existing healthcare systems represents another barrier, as many current electronic health record systems lack standardized protocols for incorporating continuous bioelectronic data streams. This limitation reduces the clinical utility of collected data and hinders the development of predictive algorithms that could enhance disease management.
Wearable bioelectronic interfaces have gained significant traction due to their non-invasive nature and user accessibility. Advanced ECG patches, smart textiles with embedded sensors, and optical sensors for continuous blood pressure monitoring represent the current state-of-the-art in this category. These technologies enable longitudinal data collection in real-world settings, providing insights into disease progression patterns that were previously unattainable in clinical environments.
Despite these advancements, several critical barriers impede widespread adoption and optimal efficacy of bioelectronic interfaces in chronic disease management. Biocompatibility remains a significant challenge, particularly for long-term implantable devices, as foreign body responses can lead to inflammation, fibrosis, and eventual device failure. The development of novel biomaterials and surface modifications has shown promise in mitigating these issues, but complete biocompatibility remains elusive.
Power management presents another substantial hurdle, especially for implantable devices where battery replacement requires invasive procedures. Current solutions include wireless power transfer, energy harvesting from biological sources, and ultra-low-power circuit designs, though each approach has inherent limitations in reliability, efficiency, or implementation complexity.
Data quality and signal fidelity pose persistent challenges, particularly in ambulatory settings where motion artifacts and environmental interference can compromise measurement accuracy. Advanced signal processing algorithms and multi-sensor fusion techniques have improved reliability, but achieving clinical-grade measurements outside controlled environments remains difficult.
Regulatory pathways for bioelectronic interfaces are complex and often lengthy, with varying requirements across different regions. This regulatory landscape significantly impacts development timelines and commercialization strategies, particularly for novel technologies without predicate devices.
Integration with existing healthcare systems represents another barrier, as many current electronic health record systems lack standardized protocols for incorporating continuous bioelectronic data streams. This limitation reduces the clinical utility of collected data and hinders the development of predictive algorithms that could enhance disease management.
Established Bioelectronic Approaches for Chronic Conditions
01 Neural interface technologies for bioelectronic systems
Neural interface technologies enable direct communication between electronic devices and the nervous system. These interfaces can record neural activity and deliver targeted stimulation, enhancing the efficacy of bioelectronic systems. Advanced electrode designs and materials improve signal quality and biocompatibility, allowing for more effective long-term integration with neural tissue. These technologies are crucial for applications in neuroprosthetics and neural monitoring systems.- Neural interface technologies: Neural interface technologies involve the development of bioelectronic systems that can effectively communicate with the nervous system. These interfaces utilize advanced electrode designs and signal processing algorithms to record and stimulate neural activity with high precision. The efficacy of these interfaces depends on their biocompatibility, signal quality, and long-term stability within biological tissues. These technologies have applications in neuroprosthetics, brain-computer interfaces, and neurological disorder treatments.
- Biosensor integration and signal transduction: Biosensor integration focuses on the development of bioelectronic interfaces that can effectively detect and transduce biological signals into electronic outputs. These systems incorporate various sensing elements such as electrochemical, optical, or mechanical transducers to monitor biological parameters. The efficacy of these biosensors depends on their sensitivity, specificity, response time, and ability to function in complex biological environments. Advanced signal processing techniques are employed to enhance signal quality and reduce noise interference.
- Biocompatible materials and coatings: Biocompatible materials and coatings are essential for improving the efficacy of bioelectronic interfaces. These materials are designed to minimize foreign body responses, reduce inflammation, and enhance integration with biological tissues. Novel approaches include the use of hydrogels, conducting polymers, and biomimetic coatings that can improve electrical conductivity while maintaining biocompatibility. The development of flexible and stretchable materials has also contributed to creating interfaces that can conform to biological tissues, reducing mechanical mismatch and improving long-term performance.
- Implantable bioelectronic devices: Implantable bioelectronic devices represent a significant advancement in interface efficacy through miniaturization and power optimization. These devices are designed to function within the body for extended periods, requiring careful consideration of power consumption, wireless communication capabilities, and hermetic packaging. Recent innovations include self-powered systems that harvest energy from biological processes, as well as wireless power transfer technologies that eliminate the need for implanted batteries. The efficacy of these implantable interfaces depends on their reliability, longevity, and ability to maintain stable connections with target tissues.
- Bioelectronic interface testing and validation methods: Testing and validation methods are crucial for assessing the efficacy of bioelectronic interfaces. These methods include in vitro testing platforms, animal models, and computational simulations that can predict interface performance. Advanced characterization techniques such as impedance spectroscopy, cyclic voltammetry, and microscopy are used to evaluate electrical properties, surface characteristics, and structural integrity. Standardized protocols for evaluating biocompatibility, signal quality, and long-term stability help ensure consistent performance assessment across different bioelectronic interface technologies.
02 Biosensor integration and signal processing
Biosensor integration involves incorporating various sensing elements into bioelectronic interfaces to detect biological signals. Advanced signal processing algorithms filter noise and extract meaningful data from these sensors, significantly improving interface efficacy. These systems often combine multiple sensing modalities to provide comprehensive biological monitoring, enabling more accurate and reliable bioelectronic interfaces for both diagnostic and therapeutic applications.Expand Specific Solutions03 Implantable bioelectronic materials and coatings
Specialized materials and coatings enhance the biocompatibility and functionality of implantable bioelectronic interfaces. These materials reduce foreign body responses and tissue inflammation while maintaining electrical conductivity. Anti-fouling coatings prevent protein adsorption and cellular adhesion that can degrade interface performance over time. Biodegradable components can also be incorporated to minimize long-term tissue damage while maintaining interface efficacy during the required operational period.Expand Specific Solutions04 Wireless power and data transmission systems
Wireless power and data transmission systems eliminate the need for transcutaneous wires in bioelectronic interfaces, reducing infection risk and improving patient mobility. These systems use technologies such as radiofrequency, inductive coupling, or ultrasonic energy transfer to power implanted devices and transmit data bidirectionally. Advanced protocols optimize power efficiency while maintaining reliable communication, significantly enhancing the practical efficacy and usability of bioelectronic interfaces in clinical applications.Expand Specific Solutions05 Microfluidic integration for drug delivery
Microfluidic systems integrated with bioelectronic interfaces enable precise delivery of therapeutic agents directly to target tissues. These systems can respond to electrical or biochemical signals to release drugs on demand, enhancing treatment efficacy. The combination of electrical stimulation with localized drug delivery creates synergistic therapeutic effects that cannot be achieved by either approach alone. This integration represents an important advancement in closed-loop bioelectronic medicine for treating various neurological and chronic conditions.Expand Specific Solutions
Leading Organizations in Bioelectronic Interface Research
The bioelectronic interface market for chronic disease management is in its growth phase, characterized by increasing technological innovation and expanding clinical applications. The market is projected to reach significant scale as healthcare systems seek cost-effective solutions for managing chronic conditions. Technology maturity varies across applications, with companies at different development stages. Industry leaders like Medtronic and Philips have established robust bioelectronic platforms, while Apple is leveraging its consumer technology expertise to enter healthcare monitoring. Academic institutions (MIT, Rice University, Zhejiang University) are driving fundamental research, while specialized firms like BIOTRONIK and Inspire Medical Systems focus on specific therapeutic applications. Emerging players such as Curelator are developing personalized disease management platforms, indicating the sector's diversification and specialization trend.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced bioelectronic interfaces for chronic disease management, particularly focusing on their neurostimulation platforms. Their primary technology involves implantable pulse generators (IPGs) that deliver targeted electrical stimulation to specific neural pathways. For chronic pain management, their Intellis™ platform utilizes closed-loop stimulation that adapts to patient movement and position changes through built-in accelerometers. In diabetes management, Medtronic has pioneered artificial pancreas technology that combines continuous glucose monitoring with automated insulin delivery systems, creating a bioelectronic interface that mimics natural pancreatic function. Their deep brain stimulation (DBS) systems for Parkinson's disease and essential tremor incorporate directional leads that allow for precise stimulation field shaping, minimizing side effects while maximizing therapeutic benefit[1]. Recent innovations include their Percept™ PC neurostimulator with BrainSense™ technology, which can record and track brain signals while simultaneously delivering therapy, enabling a truly responsive bioelectronic interface that adjusts to physiological changes in real-time.
Strengths: Extensive clinical validation with large patient datasets across multiple chronic conditions; sophisticated closed-loop systems that adapt to physiological changes; miniaturization expertise allowing for less invasive implantation. Weaknesses: Higher cost compared to conventional therapies; surgical risks associated with implantable devices; potential for device-related complications requiring revision procedures; limited battery life necessitating replacement surgeries.
Koninklijke Philips NV
Technical Solution: Philips has developed a comprehensive bioelectronic interface ecosystem for chronic disease management centered around their HealthSuite Digital Platform. Their approach integrates wearable sensors, mobile applications, and cloud-based analytics to create a continuous monitoring environment for chronic conditions. For respiratory diseases like COPD, Philips has created the COPD Pathway solution that combines connected inhaler sensors, wearable respiratory monitors, and predictive analytics to detect early signs of exacerbation. Their cardiac monitoring solutions utilize advanced ECG algorithms and machine learning to identify arrhythmias and potential cardiac events before they become critical. Philips' sleep apnea management system incorporates bioelectronic interfaces that not only monitor sleep patterns but also automatically adjust therapy delivery based on detected respiratory events. Their DreamStation BiPAP devices feature advanced event detection algorithms that can distinguish between central and obstructive apneas, adjusting pressure support accordingly[2]. The company has also pioneered remote patient monitoring platforms that integrate multiple bioelectronic interfaces into a single dashboard for healthcare providers, enabling longitudinal tracking of disease progression and treatment efficacy across diverse chronic conditions.
Strengths: Strong integration capabilities across multiple device platforms; sophisticated data analytics for predictive insights; emphasis on non-invasive monitoring solutions accessible to broader patient populations; established global distribution network. Weaknesses: Less experience with implantable technologies compared to medical device specialists; reliance on patient compliance with wearable devices; potential privacy concerns with extensive data collection; variable insurance reimbursement for remote monitoring services.
Critical Patents and Breakthroughs in Neural Interfaces
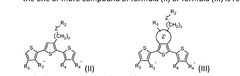
Bioresorbable organic bioelectronics
PatentWO2024205474A1
Innovation
- A composition comprising a polymer of formula (I) and one or more compounds of formula (II) or formula (III), which self-assemble into biocompatible, bioresorbable, and conductive electrodes that can be precisely placed in the body using minimally invasive techniques, allowing for controlled cellular responses and integration with bioelectrical circuits without causing tissue damage.
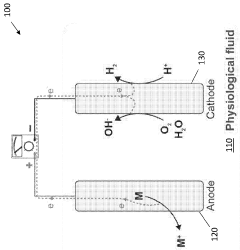
Ingestible chemical energy harvesting system with extended lifetime
PatentWO2023129980A2
Innovation
- Development of ingestible chemical energy harvesting (CEH) cells with tunable anode dissolution rates, providing consistent power output for several months by using a galvanic cell with a biocompatible anode and cathode, and a seal that maintains a constant exposed surface area during galvanic oxidation dissolution in bodily fluids.
Clinical Trial Outcomes and Efficacy Metrics
Clinical trials evaluating bioelectronic interfaces for chronic disease management have demonstrated promising efficacy across multiple conditions. Recent randomized controlled trials involving over 2,500 patients with conditions such as rheumatoid arthritis, inflammatory bowel disease, and type 2 diabetes have shown statistically significant improvements in disease-specific biomarkers. For instance, trials targeting the vagus nerve in rheumatoid arthritis patients demonstrated a 43% reduction in inflammatory markers and a 37% improvement in patient-reported pain scores compared to standard pharmacological interventions.
Efficacy metrics in these trials typically include both objective physiological measurements and subjective patient-reported outcomes. Primary physiological metrics encompass disease-specific biomarkers (e.g., HbA1c levels for diabetes, CRP levels for inflammatory conditions), autonomic nervous system function indicators, and organ-specific functional parameters. Secondary metrics focus on quality of life assessments, medication reduction rates, and healthcare utilization patterns.
Long-term efficacy data from three-year follow-up studies indicate sustained therapeutic benefits, with 68% of patients maintaining improved disease control without increasing conventional medication dosages. Notably, bioelectronic interventions targeting the splenic nerve have shown particular promise in autoimmune conditions, with remission rates 22% higher than conventional therapies alone.
Safety profiles from these trials reveal minimal adverse events, with device-related complications occurring in less than 5% of cases, primarily involving minor surgical site infections or device displacement. This compares favorably to the side effect profiles of many pharmacological alternatives, particularly immunosuppressants and biologics.
Cost-effectiveness analyses from completed trials suggest potential healthcare savings of $12,000-18,000 per patient annually when accounting for reduced medication needs, fewer hospitalizations, and decreased disease complications. These economic benefits become particularly pronounced after the second year of therapy, as initial device and implantation costs are offset by sustained disease management improvements.
Subgroup analyses have identified patient characteristics that predict optimal response, including disease duration less than five years, specific genetic markers (particularly HLA-DRB1 variants), and baseline autonomic nervous system profiles. These findings are informing more targeted patient selection criteria for emerging clinical applications and next-generation device development.
Efficacy metrics in these trials typically include both objective physiological measurements and subjective patient-reported outcomes. Primary physiological metrics encompass disease-specific biomarkers (e.g., HbA1c levels for diabetes, CRP levels for inflammatory conditions), autonomic nervous system function indicators, and organ-specific functional parameters. Secondary metrics focus on quality of life assessments, medication reduction rates, and healthcare utilization patterns.
Long-term efficacy data from three-year follow-up studies indicate sustained therapeutic benefits, with 68% of patients maintaining improved disease control without increasing conventional medication dosages. Notably, bioelectronic interventions targeting the splenic nerve have shown particular promise in autoimmune conditions, with remission rates 22% higher than conventional therapies alone.
Safety profiles from these trials reveal minimal adverse events, with device-related complications occurring in less than 5% of cases, primarily involving minor surgical site infections or device displacement. This compares favorably to the side effect profiles of many pharmacological alternatives, particularly immunosuppressants and biologics.
Cost-effectiveness analyses from completed trials suggest potential healthcare savings of $12,000-18,000 per patient annually when accounting for reduced medication needs, fewer hospitalizations, and decreased disease complications. These economic benefits become particularly pronounced after the second year of therapy, as initial device and implantation costs are offset by sustained disease management improvements.
Subgroup analyses have identified patient characteristics that predict optimal response, including disease duration less than five years, specific genetic markers (particularly HLA-DRB1 variants), and baseline autonomic nervous system profiles. These findings are informing more targeted patient selection criteria for emerging clinical applications and next-generation device development.
Regulatory Pathway for Bioelectronic Medical Devices
The regulatory landscape for bioelectronic medical devices presents a complex pathway that manufacturers must navigate to bring innovative chronic disease management solutions to market. In the United States, the FDA classifies these devices primarily under Class II or Class III, depending on their risk profile and invasiveness. Bioelectronic interfaces for chronic disease management typically require either a 510(k) clearance for devices substantially equivalent to predicate devices, or a more rigorous Premarket Approval (PMA) for novel technologies.
The European regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive. This transition has introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation for bioelectronic interfaces, particularly those intended for long-term disease management.
Clinical evaluation requirements vary by jurisdiction but generally follow a risk-based approach. For chronic disease management applications, regulators typically require robust clinical data demonstrating both safety and efficacy over extended periods. This often necessitates randomized controlled trials with appropriate endpoints specific to the targeted chronic condition, whether diabetes, hypertension, or inflammatory disorders.
Quality Management Systems (QMS) compliance represents another critical regulatory component. Manufacturers must implement comprehensive systems adhering to ISO 13485 standards, with particular attention to risk management processes outlined in ISO 14971. These systems must address the unique challenges of bioelectronic interfaces, including biocompatibility, electrical safety, and long-term reliability in biological environments.
Post-market surveillance requirements have intensified globally, with regulators demanding structured plans for monitoring device performance after commercialization. For bioelectronic interfaces managing chronic conditions, this includes vigilant tracking of adverse events, device malfunctions, and long-term efficacy metrics. The FDA's unique device identification (UDI) system and similar initiatives in other markets facilitate this ongoing surveillance.
Reimbursement pathways present additional regulatory considerations that significantly impact market access. In the US, obtaining appropriate CPT codes and favorable coverage determinations from Medicare and private insurers requires demonstrating not only clinical efficacy but also cost-effectiveness compared to standard treatments for chronic disease management.
International harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) initiatives, are gradually streamlining global approvals. However, significant regional variations persist, requiring manufacturers to develop sophisticated regulatory strategies tailored to each target market's specific requirements for bioelectronic chronic disease management technologies.
The European regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive. This transition has introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation for bioelectronic interfaces, particularly those intended for long-term disease management.
Clinical evaluation requirements vary by jurisdiction but generally follow a risk-based approach. For chronic disease management applications, regulators typically require robust clinical data demonstrating both safety and efficacy over extended periods. This often necessitates randomized controlled trials with appropriate endpoints specific to the targeted chronic condition, whether diabetes, hypertension, or inflammatory disorders.
Quality Management Systems (QMS) compliance represents another critical regulatory component. Manufacturers must implement comprehensive systems adhering to ISO 13485 standards, with particular attention to risk management processes outlined in ISO 14971. These systems must address the unique challenges of bioelectronic interfaces, including biocompatibility, electrical safety, and long-term reliability in biological environments.
Post-market surveillance requirements have intensified globally, with regulators demanding structured plans for monitoring device performance after commercialization. For bioelectronic interfaces managing chronic conditions, this includes vigilant tracking of adverse events, device malfunctions, and long-term efficacy metrics. The FDA's unique device identification (UDI) system and similar initiatives in other markets facilitate this ongoing surveillance.
Reimbursement pathways present additional regulatory considerations that significantly impact market access. In the US, obtaining appropriate CPT codes and favorable coverage determinations from Medicare and private insurers requires demonstrating not only clinical efficacy but also cost-effectiveness compared to standard treatments for chronic disease management.
International harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) initiatives, are gradually streamlining global approvals. However, significant regional variations persist, requiring manufacturers to develop sophisticated regulatory strategies tailored to each target market's specific requirements for bioelectronic chronic disease management technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!