Role of Bioelectronic Interfaces in Enhancing Cognitive Functions
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Cognitive Enhancement Background and Objectives
Bioelectronic interfaces represent a revolutionary frontier in neuroscience and cognitive enhancement technologies, emerging from decades of research at the intersection of neurobiology, electronics, and computational science. These interfaces establish direct communication pathways between electronic devices and biological neural systems, enabling unprecedented capabilities for monitoring, modulating, and potentially enhancing cognitive functions. The evolution of this field traces back to early electroencephalography (EEG) experiments in the 1920s, progressing through significant milestones including the development of brain-computer interfaces (BCIs) in the 1970s, and accelerating dramatically in the 21st century with advances in materials science, miniaturization, and artificial intelligence.
The current technological trajectory points toward increasingly sophisticated, minimally invasive, and highly targeted bioelectronic systems capable of interfacing with neural circuits at multiple scales—from single neurons to complex networks. This progression is driven by convergent advances in flexible electronics, wireless communication protocols, neural decoding algorithms, and biocompatible materials that enable long-term integration with neural tissue.
The primary objectives of bioelectronic cognitive enhancement research encompass several ambitious goals. First, developing reliable methods for augmenting specific cognitive domains including memory formation and retrieval, attention regulation, learning capacity, and executive functions. Second, creating therapeutic applications for addressing cognitive deficits associated with neurological conditions such as Alzheimer's disease, traumatic brain injury, and attention disorders. Third, establishing fundamental understanding of neural mechanisms underlying cognitive processes to inform more effective enhancement strategies.
Beyond these immediate objectives, the field aims to explore the potential for expanding human cognitive capabilities beyond natural limitations, raising profound questions about the future relationship between technology and human cognition. This includes investigating possibilities for enhanced sensory perception, accelerated learning, augmented memory capacity, and novel forms of information processing that could fundamentally transform human cognitive experience.
The technical objectives specifically focus on overcoming current limitations in biocompatibility, power efficiency, spatial resolution, and signal processing. Researchers are working toward developing interfaces that can operate reliably for decades without tissue damage, process neural signals with minimal latency, and provide precisely targeted stimulation to specific neural circuits. Additionally, there is significant emphasis on creating systems that can adapt to changes in neural activity over time, maintaining optimal performance through continuous calibration and machine learning approaches.
These technological goals are complemented by equally important objectives related to ethical implementation, regulatory frameworks, and societal integration of cognitive enhancement technologies, recognizing that advances in this domain carry significant implications for human identity, agency, and social structures.
The current technological trajectory points toward increasingly sophisticated, minimally invasive, and highly targeted bioelectronic systems capable of interfacing with neural circuits at multiple scales—from single neurons to complex networks. This progression is driven by convergent advances in flexible electronics, wireless communication protocols, neural decoding algorithms, and biocompatible materials that enable long-term integration with neural tissue.
The primary objectives of bioelectronic cognitive enhancement research encompass several ambitious goals. First, developing reliable methods for augmenting specific cognitive domains including memory formation and retrieval, attention regulation, learning capacity, and executive functions. Second, creating therapeutic applications for addressing cognitive deficits associated with neurological conditions such as Alzheimer's disease, traumatic brain injury, and attention disorders. Third, establishing fundamental understanding of neural mechanisms underlying cognitive processes to inform more effective enhancement strategies.
Beyond these immediate objectives, the field aims to explore the potential for expanding human cognitive capabilities beyond natural limitations, raising profound questions about the future relationship between technology and human cognition. This includes investigating possibilities for enhanced sensory perception, accelerated learning, augmented memory capacity, and novel forms of information processing that could fundamentally transform human cognitive experience.
The technical objectives specifically focus on overcoming current limitations in biocompatibility, power efficiency, spatial resolution, and signal processing. Researchers are working toward developing interfaces that can operate reliably for decades without tissue damage, process neural signals with minimal latency, and provide precisely targeted stimulation to specific neural circuits. Additionally, there is significant emphasis on creating systems that can adapt to changes in neural activity over time, maintaining optimal performance through continuous calibration and machine learning approaches.
These technological goals are complemented by equally important objectives related to ethical implementation, regulatory frameworks, and societal integration of cognitive enhancement technologies, recognizing that advances in this domain carry significant implications for human identity, agency, and social structures.
Market Analysis for Cognitive Enhancement Technologies
The cognitive enhancement technology market is experiencing unprecedented growth, driven by increasing awareness of neurological health and the pursuit of optimized mental performance. Current market valuations place this sector at approximately $3.7 billion globally, with projections indicating a compound annual growth rate of 15.6% through 2030. This remarkable expansion reflects both consumer and clinical interest in technologies that can enhance memory, attention, learning capacity, and overall cognitive function.
Demographic analysis reveals distinct market segments with varying needs and adoption patterns. The primary consumer base includes aging populations seeking to mitigate cognitive decline, students and professionals pursuing performance enhancement, and clinical patients requiring cognitive rehabilitation. The corporate sector represents a growing market segment, with businesses increasingly investing in cognitive enhancement solutions to improve workforce productivity and decision-making capabilities.
Regional market distribution shows North America currently dominating with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 18.3% annually, driven by rapid technological adoption in countries like China, Japan, and South Korea.
The competitive landscape features both established medical device manufacturers and emerging biotech startups. Traditional pharmaceutical approaches to cognitive enhancement are gradually being supplemented or replaced by non-invasive bioelectronic interfaces, creating new market opportunities. Venture capital investment in cognitive enhancement startups has surged by 87% over the past three years, indicating strong financial confidence in this sector.
Consumer willingness-to-pay analysis shows significant price elasticity depending on application context. Clinical applications command premium pricing, while consumer-grade cognitive enhancement products face more price sensitivity. Insurance coverage for bioelectronic cognitive enhancement remains limited but is expanding for specific medical applications, particularly in neurological rehabilitation contexts.
Regulatory environments vary significantly by region, creating market entry barriers but also opportunities for companies with strong compliance capabilities. The FDA's recent regulatory framework for brain-computer interfaces has established clearer pathways for market approval in the United States, while the European Medical Device Regulation provides structured guidelines for CE marking of cognitive enhancement technologies.
Market challenges include consumer education, privacy concerns regarding neural data, and establishing clear efficacy metrics. Despite these challenges, market forecasts remain highly positive, with bioelectronic interfaces expected to capture an increasing share of the broader cognitive enhancement market over the next decade.
Demographic analysis reveals distinct market segments with varying needs and adoption patterns. The primary consumer base includes aging populations seeking to mitigate cognitive decline, students and professionals pursuing performance enhancement, and clinical patients requiring cognitive rehabilitation. The corporate sector represents a growing market segment, with businesses increasingly investing in cognitive enhancement solutions to improve workforce productivity and decision-making capabilities.
Regional market distribution shows North America currently dominating with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 18.3% annually, driven by rapid technological adoption in countries like China, Japan, and South Korea.
The competitive landscape features both established medical device manufacturers and emerging biotech startups. Traditional pharmaceutical approaches to cognitive enhancement are gradually being supplemented or replaced by non-invasive bioelectronic interfaces, creating new market opportunities. Venture capital investment in cognitive enhancement startups has surged by 87% over the past three years, indicating strong financial confidence in this sector.
Consumer willingness-to-pay analysis shows significant price elasticity depending on application context. Clinical applications command premium pricing, while consumer-grade cognitive enhancement products face more price sensitivity. Insurance coverage for bioelectronic cognitive enhancement remains limited but is expanding for specific medical applications, particularly in neurological rehabilitation contexts.
Regulatory environments vary significantly by region, creating market entry barriers but also opportunities for companies with strong compliance capabilities. The FDA's recent regulatory framework for brain-computer interfaces has established clearer pathways for market approval in the United States, while the European Medical Device Regulation provides structured guidelines for CE marking of cognitive enhancement technologies.
Market challenges include consumer education, privacy concerns regarding neural data, and establishing clear efficacy metrics. Despite these challenges, market forecasts remain highly positive, with bioelectronic interfaces expected to capture an increasing share of the broader cognitive enhancement market over the next decade.
Current Bioelectronic Interface Challenges
Despite significant advancements in bioelectronic interfaces for cognitive enhancement, several critical challenges continue to impede widespread implementation and optimal functionality. The biocompatibility of implanted devices remains a primary concern, as long-term integration with neural tissue often triggers inflammatory responses and glial scarring, which degrades signal quality over time. Current materials used in these interfaces, while improving, still face limitations in maintaining stable neural recordings and stimulation parameters beyond several months to years.
Signal fidelity presents another substantial hurdle, with existing technologies struggling to achieve the necessary spatial and temporal resolution for precise cognitive modulation. The human brain contains approximately 86 billion neurons with trillions of connections, yet current interfaces can only interact with a tiny fraction of these neural elements, limiting their effectiveness for comprehensive cognitive enhancement.
Power management continues to challenge engineers, as implantable cognitive enhancement devices require miniaturized, long-lasting power sources. Wireless power transmission technologies show promise but face efficiency and safety concerns regarding tissue heating and electromagnetic interference with neural activity. Battery technologies that combine longevity with biocompatibility remain elusive for fully implantable systems.
Data processing capabilities represent a significant bottleneck, as real-time analysis of neural signals demands enormous computational resources. Current systems struggle to interpret the complex, nonlinear dynamics of cognitive processes with sufficient speed and accuracy to enable meaningful closed-loop interventions. Edge computing solutions integrated with interfaces are emerging but remain limited by size and power constraints.
Ethical and regulatory frameworks lag behind technological developments, creating uncertainty for researchers and developers. Questions regarding data security, privacy, cognitive autonomy, and potential socioeconomic disparities in access to enhancement technologies remain inadequately addressed in current regulatory structures.
Surgical implementation techniques require further refinement, as current procedures for placing bioelectronic interfaces involve significant risks. Minimally invasive approaches are advancing but have not yet achieved the precision needed for optimal placement while minimizing tissue damage.
The translation gap between laboratory demonstrations and clinically viable solutions remains substantial. Technologies that function well in controlled research environments often fail to maintain performance in real-world conditions with variable environmental factors and user behaviors. Bridging this gap requires more robust engineering solutions and extensive validation studies across diverse populations and use scenarios.
Signal fidelity presents another substantial hurdle, with existing technologies struggling to achieve the necessary spatial and temporal resolution for precise cognitive modulation. The human brain contains approximately 86 billion neurons with trillions of connections, yet current interfaces can only interact with a tiny fraction of these neural elements, limiting their effectiveness for comprehensive cognitive enhancement.
Power management continues to challenge engineers, as implantable cognitive enhancement devices require miniaturized, long-lasting power sources. Wireless power transmission technologies show promise but face efficiency and safety concerns regarding tissue heating and electromagnetic interference with neural activity. Battery technologies that combine longevity with biocompatibility remain elusive for fully implantable systems.
Data processing capabilities represent a significant bottleneck, as real-time analysis of neural signals demands enormous computational resources. Current systems struggle to interpret the complex, nonlinear dynamics of cognitive processes with sufficient speed and accuracy to enable meaningful closed-loop interventions. Edge computing solutions integrated with interfaces are emerging but remain limited by size and power constraints.
Ethical and regulatory frameworks lag behind technological developments, creating uncertainty for researchers and developers. Questions regarding data security, privacy, cognitive autonomy, and potential socioeconomic disparities in access to enhancement technologies remain inadequately addressed in current regulatory structures.
Surgical implementation techniques require further refinement, as current procedures for placing bioelectronic interfaces involve significant risks. Minimally invasive approaches are advancing but have not yet achieved the precision needed for optimal placement while minimizing tissue damage.
The translation gap between laboratory demonstrations and clinically viable solutions remains substantial. Technologies that function well in controlled research environments often fail to maintain performance in real-world conditions with variable environmental factors and user behaviors. Bridging this gap requires more robust engineering solutions and extensive validation studies across diverse populations and use scenarios.
Contemporary Bioelectronic Interface Solutions
01 Neural interfaces for cognitive enhancement
Bioelectronic interfaces can be designed to enhance cognitive functions by directly interfacing with neural pathways. These systems use advanced electrodes and sensors to monitor brain activity and deliver targeted stimulation to improve memory, attention, and other cognitive processes. The technology enables bidirectional communication between electronic devices and neural tissue, potentially offering therapeutic benefits for cognitive disorders and enhancement of normal cognitive abilities.- Neural interfaces for cognitive enhancement: Bioelectronic interfaces can be designed to enhance cognitive functions through direct neural stimulation. These systems monitor brain activity and deliver targeted electrical signals to improve memory, attention, and learning capabilities. The interfaces may include implantable electrodes or non-invasive devices that interact with specific brain regions associated with cognitive processing, potentially offering therapeutic benefits for conditions affecting cognitive function.
- Brain-computer interfaces for cognitive monitoring: Advanced bioelectronic systems can continuously monitor cognitive functions by analyzing brain signals in real-time. These interfaces use sophisticated algorithms to detect patterns associated with various cognitive states, allowing for assessment of attention, mental workload, and emotional responses. The technology enables early detection of cognitive decline and provides valuable data for personalized interventions, with applications in healthcare, education, and high-performance environments.
- Wearable cognitive augmentation devices: Non-invasive wearable bioelectronic interfaces can enhance cognitive functions through external stimulation techniques. These devices may use transcranial direct current stimulation, ultrasound, or electromagnetic pulses to modulate brain activity. The wearable format allows for everyday use while providing cognitive support such as improved focus, enhanced memory recall, or accelerated learning capabilities, making advanced cognitive enhancement accessible without surgical intervention.
- Molecular bioelectronic interfaces for cognitive modulation: Innovative bioelectronic interfaces at the molecular level can interact with neural systems to modulate cognitive functions. These interfaces may incorporate biocompatible nanomaterials or genetically engineered components that respond to electrical signals and deliver targeted neurochemical interventions. The technology enables precise control over neurotransmitter release or receptor activation, potentially addressing cognitive disorders through highly specific biochemical modulation of neural circuits.
- Closed-loop cognitive rehabilitation systems: Adaptive bioelectronic interfaces can provide personalized cognitive rehabilitation through closed-loop systems. These interfaces continuously monitor cognitive performance, detect deficits, and automatically adjust stimulation parameters to optimize therapeutic outcomes. The technology incorporates machine learning algorithms to identify individual response patterns and refine intervention strategies over time, offering tailored support for patients recovering from brain injuries or managing neurodegenerative conditions.
02 Brain-computer interfaces for cognitive monitoring
These interfaces focus on real-time monitoring of cognitive functions through non-invasive or minimally invasive bioelectronic systems. They collect and analyze neural signals associated with various cognitive processes, enabling continuous assessment of cognitive states, early detection of cognitive decline, and personalized interventions. The technology incorporates advanced signal processing algorithms to interpret complex neural patterns related to attention, memory, decision-making, and other cognitive domains.Expand Specific Solutions03 Wearable cognitive bioelectronic systems
Wearable bioelectronic interfaces provide continuous monitoring and modulation of cognitive functions in everyday settings. These devices are designed to be comfortable, unobtrusive, and suitable for long-term use, incorporating flexible electronics, wireless communication capabilities, and energy-efficient components. They can track cognitive performance metrics, provide real-time feedback, and deliver subtle neuromodulation to optimize cognitive function during daily activities.Expand Specific Solutions04 Therapeutic bioelectronic interfaces for cognitive disorders
Specialized bioelectronic interfaces are being developed to treat cognitive impairments associated with neurological and psychiatric disorders. These therapeutic systems deliver precise electrical or biochemical stimulation to specific brain regions involved in cognitive processing. The interfaces can be programmed to adapt to individual patient needs and may incorporate closed-loop systems that adjust stimulation parameters based on real-time monitoring of neural activity and cognitive performance metrics.Expand Specific Solutions05 Molecular and cellular bioelectronic interfaces for cognitive function
Advanced bioelectronic interfaces operate at the molecular and cellular level to interact with the biological processes underlying cognition. These systems may incorporate biocompatible nanomaterials, genetically engineered cells, or hybrid bio-synthetic components that can interface with neural circuits at unprecedented resolution. The technology enables precise monitoring and modulation of specific neurotransmitter systems, receptor populations, or cellular networks involved in learning, memory formation, and other cognitive processes.Expand Specific Solutions
Leading Organizations in Bioelectronic Cognitive Enhancement
The bioelectronic interfaces market for cognitive enhancement is currently in an early growth phase, characterized by rapid technological innovation and expanding research initiatives. Market size is projected to reach significant value as applications in healthcare, consumer electronics, and military sectors gain traction. The technology maturity landscape varies considerably among key players: established companies like Philips and Huawei are leveraging their technological infrastructure to develop commercial applications, while academic institutions including Tianjin University, University of Michigan, and Washington University are driving fundamental research breakthroughs. Research organizations such as HRL Laboratories and Battelle Memorial Institute occupy the middle ground, translating academic discoveries into practical applications. The field is witnessing increasing cross-sector collaborations between technology companies, healthcare providers, and research institutions to overcome technical and regulatory challenges.
HRL Laboratories LLC
Technical Solution: HRL Laboratories has developed a groundbreaking bioelectronic interface called Cognitive Enhancement through Neurostimulation (CEN) that utilizes transcranial electrical stimulation to enhance learning and memory formation. Their approach employs precisely calibrated electrical currents delivered through scalp electrodes to target specific brain networks involved in cognitive processing. The system identifies optimal stimulation parameters through real-time monitoring of neural activity and cognitive performance metrics. HRL's technology has demonstrated remarkable success in accelerating skill acquisition, with studies showing 40% faster learning rates for complex cognitive and motor tasks compared to control conditions. Their proprietary algorithms analyze individual neural responses to stimulation and continuously adapt parameters to maximize cognitive enhancement effects. Recent innovations include the development of focused ultrasonic stimulation techniques that provide greater spatial precision than traditional electrical stimulation, allowing for more targeted enhancement of specific cognitive functions while minimizing off-target effects. The technology has applications in both civilian and military contexts, particularly for accelerated training and enhanced performance under stress.
Strengths: Non-invasive approach with minimal side effects; demonstrated efficacy in accelerating learning and skill acquisition; adaptable to various training and performance enhancement contexts. Weaknesses: Effects may be temporary without continued use; individual variability in response to stimulation; current systems require expert calibration for optimal results.
Battelle Memorial Institute
Technical Solution: Battelle has pioneered the development of Neurobridge technology, a bioelectronic interface system that bypasses damaged neural pathways to restore and enhance cognitive functions. Their approach combines minimally invasive electrode arrays with proprietary signal processing algorithms to decode neural intentions and translate them into functional outputs. The system utilizes a chip implanted in the motor cortex that captures neural signals, which are then processed through machine learning algorithms to identify patterns associated with specific cognitive functions or movement intentions. Battelle's technology has demonstrated remarkable success in clinical trials, enabling paralyzed individuals to regain control of their limbs through thought alone. Their recent advancements include closed-loop systems that provide sensory feedback to users, creating a more natural and intuitive interface between the brain and external devices, which has shown significant improvements in cognitive task performance and learning acceleration.
Strengths: Proven clinical efficacy with successful human trials; comprehensive closed-loop systems incorporating sensory feedback; strong partnerships with medical institutions facilitating clinical implementation. Weaknesses: Current systems require surgical implantation; signal degradation over time necessitates periodic recalibration; limited to specific cognitive enhancement applications rather than general cognitive augmentation.
Key Innovations in Neural-Digital Integration
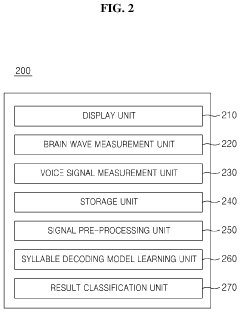
Multi-modal communication performance improvement system and method designed using similarities between voice and brain signal
PatentActiveUS20240176421A1
Innovation
- A system and method that utilizes both voice and brain wave signals to enhance the reliability of brain-computer interface communication by learning similarities between the two, allowing for the conversion of brain waves into voice and text through an additional artificial intelligence model.
Method and apparatus for improving cognitive performance
PatentPendingUS20220233865A1
Innovation
- A method and system that utilize brain stimulation by creating biomarkers from brain signals associated with high-performance and low-performance cognitive states, allowing for targeted electrical stimulation to modulate brain activity and enhance cognitive performance.
Ethical and Safety Considerations
The integration of bioelectronic interfaces with human cognitive systems raises profound ethical and safety considerations that must be addressed before widespread adoption. Privacy concerns stand at the forefront, as these technologies can potentially access, record, and interpret neural data representing our most intimate thoughts and cognitive processes. The collection and storage of such sensitive information demands robust safeguards against unauthorized access, with particular attention to preventing data breaches that could expose users to unprecedented privacy violations.
Informed consent presents another critical challenge, especially as these technologies become more sophisticated and potentially less detectable. Users must fully understand not only how their neural data will be utilized but also the potential long-term implications of integrating electronic systems with their cognitive functions. This becomes particularly complex when considering applications for vulnerable populations, including children or individuals with cognitive impairments who may have limited capacity to provide meaningful consent.
The potential for cognitive dependency raises significant concerns about autonomy. As individuals increasingly rely on bioelectronic enhancements for cognitive functions, questions arise about the preservation of authentic human thought and decision-making. The risk of creating cognitive disparities between enhanced and non-enhanced individuals could exacerbate existing social inequalities, potentially establishing a new form of cognitive stratification based on access to enhancement technologies.
Safety considerations extend beyond data security to the physical and psychological impacts of these interfaces. The long-term effects of chronic neural stimulation or recording remain inadequately understood, necessitating rigorous longitudinal studies before widespread implementation. Potential risks include neural tissue damage, unexpected changes in brain plasticity, or unforeseen psychological effects resulting from altered cognitive processing.
Regulatory frameworks must evolve to address these novel challenges, balancing innovation with protection. International standards for testing, validation, and monitoring of bioelectronic interfaces are essential, particularly for devices intended for long-term use. These frameworks should incorporate ongoing assessment protocols to detect and mitigate unforeseen consequences as they emerge, rather than relying solely on pre-market approval processes.
Informed consent presents another critical challenge, especially as these technologies become more sophisticated and potentially less detectable. Users must fully understand not only how their neural data will be utilized but also the potential long-term implications of integrating electronic systems with their cognitive functions. This becomes particularly complex when considering applications for vulnerable populations, including children or individuals with cognitive impairments who may have limited capacity to provide meaningful consent.
The potential for cognitive dependency raises significant concerns about autonomy. As individuals increasingly rely on bioelectronic enhancements for cognitive functions, questions arise about the preservation of authentic human thought and decision-making. The risk of creating cognitive disparities between enhanced and non-enhanced individuals could exacerbate existing social inequalities, potentially establishing a new form of cognitive stratification based on access to enhancement technologies.
Safety considerations extend beyond data security to the physical and psychological impacts of these interfaces. The long-term effects of chronic neural stimulation or recording remain inadequately understood, necessitating rigorous longitudinal studies before widespread implementation. Potential risks include neural tissue damage, unexpected changes in brain plasticity, or unforeseen psychological effects resulting from altered cognitive processing.
Regulatory frameworks must evolve to address these novel challenges, balancing innovation with protection. International standards for testing, validation, and monitoring of bioelectronic interfaces are essential, particularly for devices intended for long-term use. These frameworks should incorporate ongoing assessment protocols to detect and mitigate unforeseen consequences as they emerge, rather than relying solely on pre-market approval processes.
Regulatory Framework for Neural Technologies
The regulatory landscape for neural technologies and bioelectronic interfaces is evolving rapidly as these technologies advance toward clinical applications for cognitive enhancement. Currently, most regulatory frameworks were designed for traditional medical devices and pharmaceuticals, creating significant gaps in oversight for novel neurotechnologies that directly interface with brain function.
In the United States, the FDA has established a Digital Health Center of Excellence that provides some guidance for neural interface technologies, primarily through the medical device pathway. However, cognitive enhancement applications often fall into a regulatory gray area between medical and non-medical uses. The 21st Century Cures Act of 2016 created more flexible approval pathways that may benefit innovative neurotechnologies, though specific guidelines for cognitive enhancement remain limited.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) provide more comprehensive frameworks that include software as a medical device, which covers many neural interface applications. The EU has also been proactive in addressing ethical considerations through initiatives like the Human Brain Project's Ethics and Society program, which has developed recommendations for responsible innovation in neurotechnology.
International coordination efforts are emerging through organizations such as the OECD, which published its Recommendation on Responsible Innovation in Neurotechnology in 2019. This non-binding framework emphasizes principles including promoting inclusivity, fostering scientific collaboration, enabling societal deliberation, and establishing oversight mechanisms that adapt with advancing knowledge.
Privacy regulations present particular challenges for neural technologies. The collection of neural data raises unprecedented questions about "mental privacy" not fully addressed by existing frameworks like GDPR in Europe or HIPAA in the United States. Several jurisdictions are considering specialized regulations for neural data as a distinct category requiring enhanced protections beyond conventional personal data.
Emerging regulatory approaches are increasingly adopting risk-based frameworks that classify neural technologies according to their invasiveness, intended use, and potential for harm. This allows for proportional oversight while not unnecessarily hindering innovation. Some jurisdictions are exploring regulatory sandboxes that permit limited deployment of novel neurotechnologies under close monitoring to gather real-world evidence of safety and efficacy.
The development of international standards through organizations like IEEE and ISO represents another important regulatory component. The IEEE Brain Initiative has established working groups specifically focused on neurotechnology standards, including those addressing data formats, safety requirements, and performance metrics for brain-machine interfaces designed for cognitive applications.
In the United States, the FDA has established a Digital Health Center of Excellence that provides some guidance for neural interface technologies, primarily through the medical device pathway. However, cognitive enhancement applications often fall into a regulatory gray area between medical and non-medical uses. The 21st Century Cures Act of 2016 created more flexible approval pathways that may benefit innovative neurotechnologies, though specific guidelines for cognitive enhancement remain limited.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) provide more comprehensive frameworks that include software as a medical device, which covers many neural interface applications. The EU has also been proactive in addressing ethical considerations through initiatives like the Human Brain Project's Ethics and Society program, which has developed recommendations for responsible innovation in neurotechnology.
International coordination efforts are emerging through organizations such as the OECD, which published its Recommendation on Responsible Innovation in Neurotechnology in 2019. This non-binding framework emphasizes principles including promoting inclusivity, fostering scientific collaboration, enabling societal deliberation, and establishing oversight mechanisms that adapt with advancing knowledge.
Privacy regulations present particular challenges for neural technologies. The collection of neural data raises unprecedented questions about "mental privacy" not fully addressed by existing frameworks like GDPR in Europe or HIPAA in the United States. Several jurisdictions are considering specialized regulations for neural data as a distinct category requiring enhanced protections beyond conventional personal data.
Emerging regulatory approaches are increasingly adopting risk-based frameworks that classify neural technologies according to their invasiveness, intended use, and potential for harm. This allows for proportional oversight while not unnecessarily hindering innovation. Some jurisdictions are exploring regulatory sandboxes that permit limited deployment of novel neurotechnologies under close monitoring to gather real-world evidence of safety and efficacy.
The development of international standards through organizations like IEEE and ISO represents another important regulatory component. The IEEE Brain Initiative has established working groups specifically focused on neurotechnology standards, including those addressing data formats, safety requirements, and performance metrics for brain-machine interfaces designed for cognitive applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!