Standards and Regulations Impacting Bioelectronic Interface Development
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Regulatory Landscape and Objectives
Bioelectronic interfaces represent a rapidly evolving field at the intersection of electronics and biology, creating unprecedented opportunities for medical diagnostics, treatment, and human augmentation. The regulatory landscape governing these technologies is complex and multifaceted, reflecting the unique challenges posed by devices that directly interface with human biological systems. Understanding this landscape is crucial for successful development and deployment of bioelectronic technologies.
The evolution of bioelectronic interface regulations has been shaped by the gradual recognition of these technologies as a distinct category requiring specialized oversight. Initially governed by broader medical device regulations, the field has seen increasing regulatory specificity as applications have diversified and potential risks have become better understood. This evolution continues today, with regulatory frameworks adapting to accommodate emerging technologies like neural interfaces and implantable sensors.
Current regulatory objectives focus on balancing innovation with safety and ethical considerations. Primary concerns include biocompatibility, long-term stability, data security, and informed consent. Regulatory bodies worldwide are working to establish standards that address these concerns while allowing for technological advancement. The FDA's guidance on implantable bioelectronics and the EU's Medical Device Regulation (MDR) represent significant steps toward creating comprehensive regulatory frameworks.
International harmonization efforts are underway to streamline approval processes across different jurisdictions. The International Medical Device Regulators Forum (IMDRF) has been instrumental in developing globally recognized standards for bioelectronic interfaces, though significant regional variations persist. These differences can present challenges for developers seeking multi-market approval.
Technical standards development is occurring in parallel with regulatory evolution. Organizations such as IEEE, ASTM, and ISO are developing specifications for biocompatibility testing, electrical safety, and performance evaluation. These standards provide crucial benchmarks for developers and serve as reference points for regulatory compliance.
The field's regulatory objectives are increasingly incorporating ethical considerations alongside traditional safety concerns. Issues of data privacy, cognitive liberty, and equitable access are becoming central to regulatory discussions, particularly for neural interfaces with potential to influence cognition or behavior.
Looking forward, regulatory frameworks will need to evolve to address emerging capabilities such as bidirectional neural interfaces, autonomous bioelectronic systems, and devices with machine learning capabilities. Anticipatory governance approaches are being explored to ensure regulations can adapt to rapid technological change without impeding innovation.
The ultimate goal of the regulatory landscape is to facilitate responsible development of bioelectronic interfaces that can realize their transformative potential while protecting users and addressing societal concerns. Achieving this balance requires ongoing dialogue between developers, regulators, ethicists, and potential users.
The evolution of bioelectronic interface regulations has been shaped by the gradual recognition of these technologies as a distinct category requiring specialized oversight. Initially governed by broader medical device regulations, the field has seen increasing regulatory specificity as applications have diversified and potential risks have become better understood. This evolution continues today, with regulatory frameworks adapting to accommodate emerging technologies like neural interfaces and implantable sensors.
Current regulatory objectives focus on balancing innovation with safety and ethical considerations. Primary concerns include biocompatibility, long-term stability, data security, and informed consent. Regulatory bodies worldwide are working to establish standards that address these concerns while allowing for technological advancement. The FDA's guidance on implantable bioelectronics and the EU's Medical Device Regulation (MDR) represent significant steps toward creating comprehensive regulatory frameworks.
International harmonization efforts are underway to streamline approval processes across different jurisdictions. The International Medical Device Regulators Forum (IMDRF) has been instrumental in developing globally recognized standards for bioelectronic interfaces, though significant regional variations persist. These differences can present challenges for developers seeking multi-market approval.
Technical standards development is occurring in parallel with regulatory evolution. Organizations such as IEEE, ASTM, and ISO are developing specifications for biocompatibility testing, electrical safety, and performance evaluation. These standards provide crucial benchmarks for developers and serve as reference points for regulatory compliance.
The field's regulatory objectives are increasingly incorporating ethical considerations alongside traditional safety concerns. Issues of data privacy, cognitive liberty, and equitable access are becoming central to regulatory discussions, particularly for neural interfaces with potential to influence cognition or behavior.
Looking forward, regulatory frameworks will need to evolve to address emerging capabilities such as bidirectional neural interfaces, autonomous bioelectronic systems, and devices with machine learning capabilities. Anticipatory governance approaches are being explored to ensure regulations can adapt to rapid technological change without impeding innovation.
The ultimate goal of the regulatory landscape is to facilitate responsible development of bioelectronic interfaces that can realize their transformative potential while protecting users and addressing societal concerns. Achieving this balance requires ongoing dialogue between developers, regulators, ethicists, and potential users.
Market Demand Analysis for Compliant Bioelectronic Interfaces
The bioelectronic interface market is experiencing unprecedented growth, driven by increasing prevalence of neurological disorders, rising geriatric population, and growing demand for non-invasive treatment options. The global bioelectronic medicine market was valued at approximately $17.2 billion in 2021 and is projected to reach $29.6 billion by 2028, growing at a CAGR of 7.9%. This substantial growth reflects the expanding applications of bioelectronic interfaces across multiple healthcare segments.
Healthcare providers are increasingly seeking compliant bioelectronic interfaces that adhere to stringent regulatory standards while delivering effective patient outcomes. The demand is particularly strong in neurostimulation devices, with applications for chronic pain management, movement disorders, and psychiatric conditions showing significant market traction. The global neurostimulation devices market alone is expected to reach $13.3 billion by 2026.
Consumer demand for wearable bioelectronic interfaces has surged, with patients preferring devices that offer minimal invasiveness, reduced side effects, and improved quality of life compared to traditional pharmaceutical interventions. This shift is evidenced by the 35% annual increase in patient adoption of bioelectronic therapies for conditions previously treated exclusively with medications.
Regulatory compliance has emerged as a critical market differentiator, with healthcare systems and insurance providers increasingly favoring devices that meet or exceed international safety and efficacy standards. Market research indicates that 78% of healthcare procurement specialists cite regulatory compliance as a top-three decision factor when selecting bioelectronic interface technologies.
Regional market analysis reveals varying demand patterns, with North America currently dominating the market share at 42%, followed by Europe at 28% and Asia-Pacific showing the fastest growth rate at 11.2% annually. This geographic distribution correlates strongly with regulatory maturity in these regions, highlighting the interconnection between market demand and regulatory frameworks.
Industry surveys indicate that manufacturers are responding to this demand by investing heavily in regulatory expertise, with 67% of bioelectronic device companies increasing their regulatory affairs budgets by at least 15% in the past two years. This investment reflects recognition that regulatory compliance is not merely a barrier to market entry but a strategic advantage in capturing market share.
The convergence of artificial intelligence with bioelectronic interfaces is creating new market segments, with demand for smart, adaptive devices that can adjust therapy based on patient response while maintaining compliance with evolving regulations. This trend is expected to accelerate, with the AI-enhanced bioelectronic interface segment projected to grow at 14.3% annually through 2030.
Healthcare providers are increasingly seeking compliant bioelectronic interfaces that adhere to stringent regulatory standards while delivering effective patient outcomes. The demand is particularly strong in neurostimulation devices, with applications for chronic pain management, movement disorders, and psychiatric conditions showing significant market traction. The global neurostimulation devices market alone is expected to reach $13.3 billion by 2026.
Consumer demand for wearable bioelectronic interfaces has surged, with patients preferring devices that offer minimal invasiveness, reduced side effects, and improved quality of life compared to traditional pharmaceutical interventions. This shift is evidenced by the 35% annual increase in patient adoption of bioelectronic therapies for conditions previously treated exclusively with medications.
Regulatory compliance has emerged as a critical market differentiator, with healthcare systems and insurance providers increasingly favoring devices that meet or exceed international safety and efficacy standards. Market research indicates that 78% of healthcare procurement specialists cite regulatory compliance as a top-three decision factor when selecting bioelectronic interface technologies.
Regional market analysis reveals varying demand patterns, with North America currently dominating the market share at 42%, followed by Europe at 28% and Asia-Pacific showing the fastest growth rate at 11.2% annually. This geographic distribution correlates strongly with regulatory maturity in these regions, highlighting the interconnection between market demand and regulatory frameworks.
Industry surveys indicate that manufacturers are responding to this demand by investing heavily in regulatory expertise, with 67% of bioelectronic device companies increasing their regulatory affairs budgets by at least 15% in the past two years. This investment reflects recognition that regulatory compliance is not merely a barrier to market entry but a strategic advantage in capturing market share.
The convergence of artificial intelligence with bioelectronic interfaces is creating new market segments, with demand for smart, adaptive devices that can adjust therapy based on patient response while maintaining compliance with evolving regulations. This trend is expected to accelerate, with the AI-enhanced bioelectronic interface segment projected to grow at 14.3% annually through 2030.
Current Standards Framework and Technical Challenges
The bioelectronic interface development landscape is currently governed by a complex framework of standards and regulations that vary significantly across regions and applications. The International Organization for Standardization (ISO) has established several key standards, including ISO 14708 for implantable medical devices and ISO 13485 for quality management systems. These standards provide baseline requirements for safety, performance, and risk management but often lag behind the rapid pace of technological innovation in bioelectronics.
In the United States, the Food and Drug Administration (FDA) classifies bioelectronic interfaces primarily under Class II or Class III medical devices, requiring either 510(k) clearance or premarket approval (PMA). The FDA has recently introduced the Digital Health Software Precertification Program to streamline regulatory processes for software-driven medical technologies, acknowledging the unique challenges posed by rapidly evolving bioelectronic systems.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have significantly increased requirements for clinical evidence and post-market surveillance, creating substantial challenges for bioelectronic interface developers. These regulations have extended development timelines by an estimated 12-18 months and increased compliance costs by 30-40% compared to previous directives.
A critical technical challenge in the standards landscape is the lack of specific protocols for evaluating long-term biocompatibility of novel materials used in bioelectronic interfaces. Current standards like ISO 10993 for biological evaluation of medical devices were developed primarily for traditional materials and do not adequately address the unique properties of advanced materials such as conducting polymers, carbon nanotubes, or graphene-based composites.
Interoperability presents another significant challenge, with limited standardization for data formats, communication protocols, and interfaces between bioelectronic devices and existing healthcare systems. The IEEE 11073 standards family attempts to address medical device communication, but adoption remains inconsistent across manufacturers and healthcare providers.
Cybersecurity requirements represent an emerging regulatory focus, with the FDA's "Content of Premarket Submissions for Management of Cybersecurity in Medical Devices" guidance document establishing expectations for security controls. However, these guidelines often conflict with design constraints for miniaturized, low-power bioelectronic interfaces, creating tension between security requirements and practical implementation.
The fragmented regulatory landscape across different regions creates significant market entry barriers, with manufacturers needing to navigate multiple approval pathways with inconsistent requirements. This regulatory complexity disproportionately impacts smaller companies and academic institutions, potentially limiting innovation in the bioelectronic interface field.
In the United States, the Food and Drug Administration (FDA) classifies bioelectronic interfaces primarily under Class II or Class III medical devices, requiring either 510(k) clearance or premarket approval (PMA). The FDA has recently introduced the Digital Health Software Precertification Program to streamline regulatory processes for software-driven medical technologies, acknowledging the unique challenges posed by rapidly evolving bioelectronic systems.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have significantly increased requirements for clinical evidence and post-market surveillance, creating substantial challenges for bioelectronic interface developers. These regulations have extended development timelines by an estimated 12-18 months and increased compliance costs by 30-40% compared to previous directives.
A critical technical challenge in the standards landscape is the lack of specific protocols for evaluating long-term biocompatibility of novel materials used in bioelectronic interfaces. Current standards like ISO 10993 for biological evaluation of medical devices were developed primarily for traditional materials and do not adequately address the unique properties of advanced materials such as conducting polymers, carbon nanotubes, or graphene-based composites.
Interoperability presents another significant challenge, with limited standardization for data formats, communication protocols, and interfaces between bioelectronic devices and existing healthcare systems. The IEEE 11073 standards family attempts to address medical device communication, but adoption remains inconsistent across manufacturers and healthcare providers.
Cybersecurity requirements represent an emerging regulatory focus, with the FDA's "Content of Premarket Submissions for Management of Cybersecurity in Medical Devices" guidance document establishing expectations for security controls. However, these guidelines often conflict with design constraints for miniaturized, low-power bioelectronic interfaces, creating tension between security requirements and practical implementation.
The fragmented regulatory landscape across different regions creates significant market entry barriers, with manufacturers needing to navigate multiple approval pathways with inconsistent requirements. This regulatory complexity disproportionately impacts smaller companies and academic institutions, potentially limiting innovation in the bioelectronic interface field.
Compliance Strategies for Bioelectronic Interface Development
01 Neural interfaces for bioelectronic applications
Neural interfaces are a key component in bioelectronic systems, enabling direct communication between electronic devices and the nervous system. These interfaces can record neural activity and deliver stimulation to specific neural targets. Advanced materials and fabrication techniques are used to create flexible, biocompatible neural electrodes that minimize tissue damage and immune response while maintaining long-term functionality. These interfaces are crucial for applications such as neuroprosthetics, brain-computer interfaces, and neuromodulation therapies.- Neural interfaces for bioelectronic applications: Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neurons, or both, enabling applications in neural prosthetics, brain-machine interfaces, and treatment of neurological disorders. Advanced materials and fabrication techniques are used to create biocompatible electrodes that can effectively interface with neural tissue while minimizing tissue damage and inflammatory responses.
- Flexible and stretchable bioelectronic interfaces: Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic surfaces of biological tissues, providing stable long-term connections. These interfaces utilize elastic materials, serpentine structures, or mesh designs to accommodate movement while maintaining electrical functionality. Such interfaces are particularly valuable for applications requiring integration with soft tissues or organs that undergo regular movement, such as skin, heart, or brain tissue.
- Biosensing and molecular detection interfaces: Bioelectronic interfaces for biosensing applications incorporate biological recognition elements with electronic transduction mechanisms to detect specific biomolecules, cells, or physiological parameters. These interfaces may utilize enzymes, antibodies, aptamers, or other biological components to achieve high specificity. The electronic components translate biological recognition events into measurable electrical signals, enabling applications in medical diagnostics, environmental monitoring, and biomedical research.
- Implantable bioelectronic medical devices: Implantable bioelectronic interfaces are designed for long-term integration within the body to monitor physiological parameters or deliver therapeutic interventions. These devices address challenges of biocompatibility, power supply, wireless communication, and longevity in the biological environment. Advanced encapsulation methods protect electronic components from bodily fluids while allowing for effective interaction with surrounding tissues, enabling applications such as cardiac pacemakers, neural stimulators, and continuous glucose monitors.
- Nanomaterial-based bioelectronic interfaces: Nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles are incorporated into bioelectronic interfaces to enhance performance characteristics. These materials offer advantages including increased surface area, improved electrical conductivity, enhanced mechanical properties, and unique interactions with biological systems. Nanomaterial-based interfaces enable higher sensitivity in biosensing applications, more efficient signal transduction, and reduced dimensions for minimally invasive integration with biological systems.
02 Implantable bioelectronic devices
Implantable bioelectronic devices represent a significant advancement in medical technology, offering continuous monitoring and therapeutic capabilities. These devices incorporate biocompatible materials, miniaturized electronics, and power management systems designed for long-term implantation within the body. They can interface with various biological tissues and systems to monitor physiological parameters, deliver targeted therapy, or restore lost function. Key considerations include hermeticity, biocompatibility, power efficiency, and wireless communication capabilities to ensure safe and effective operation within the biological environment.Expand Specific Solutions03 Flexible and stretchable bioelectronic interfaces
Flexible and stretchable bioelectronic interfaces represent a significant advancement in creating seamless connections between rigid electronic components and soft biological tissues. These interfaces utilize innovative materials such as conductive polymers, liquid metals, and engineered composites that can bend, stretch, and conform to dynamic biological surfaces while maintaining electrical functionality. The mechanical compliance of these interfaces reduces tissue damage and inflammatory responses, enabling long-term integration with living systems. Applications include wearable health monitors, conformable neural interfaces, and implantable therapeutic devices that can move naturally with the body.Expand Specific Solutions04 Biosensing and bioelectronic detection systems
Biosensing and bioelectronic detection systems integrate biological recognition elements with electronic transducers to detect and quantify biological analytes. These systems utilize various sensing mechanisms including electrochemical, optical, and mechanical transduction to convert biological interactions into measurable electronic signals. Advanced bioelectronic interfaces enhance sensitivity, selectivity, and response time while enabling miniaturization and multiplexing capabilities. Applications include point-of-care diagnostics, continuous health monitoring, environmental sensing, and biodefense, where rapid and accurate detection of biomarkers, pathogens, or toxins is critical.Expand Specific Solutions05 Bioelectronic materials and fabrication techniques
Advanced materials and fabrication techniques are essential for creating effective bioelectronic interfaces. These include conducting polymers, carbon-based materials, and hybrid organic-inorganic composites that offer biocompatibility and electrical conductivity. Micro and nanofabrication methods such as photolithography, 3D printing, and self-assembly enable precise patterning of these materials into functional devices. Surface modification strategies enhance biocompatibility and promote specific biological interactions. These materials and techniques address challenges in creating stable, long-lasting interfaces between electronic components and biological systems while minimizing foreign body responses and maintaining functionality in physiological environments.Expand Specific Solutions
Key Regulatory Bodies and Industry Stakeholders
The bioelectronic interface development landscape is currently in a growth phase, with the market expected to reach significant expansion as technologies mature. Key players represent diverse sectors, with academic institutions like MIT, Caltech, and Tsinghua University driving fundamental research, while commercial entities including Agilent Technologies, MediaTek, and BOE Technology Group focus on practical applications. Regulatory frameworks vary globally, creating a complex compliance environment that impacts development timelines. Companies like Goodix Technology and Abbott Diabetes Care are navigating these challenges by investing in standards-compliant technologies. The field is characterized by increasing collaboration between research institutions and industry partners to address regulatory hurdles while accelerating innovation in medical-grade bioelectronic interfaces.
Massachusetts Institute of Technology
Technical Solution: MIT has developed pioneering approaches to bioelectronic interfaces that address regulatory challenges through innovative materials and design methodologies. Their research teams have created flexible, biocompatible electronic systems that conform to tissue surfaces while meeting stringent regulatory requirements. MIT's approach incorporates materials science innovations that address ISO 10993 biocompatibility standards through novel polymers and surface modifications that minimize foreign body responses[2]. Their neural interface technologies implement comprehensive risk management strategies aligned with ISO 14971, particularly addressing long-term safety concerns for chronically implanted devices. MIT researchers have developed specialized testing protocols for evaluating the electrical safety of neural stimulation parameters that exceed standard requirements in IEC 60601, establishing new benchmarks for safety evaluation[4]. The institute has pioneered minimally invasive delivery methods for bioelectronic interfaces that reduce surgical risks while maintaining compliance with regulatory frameworks for implantable devices. MIT's regulatory strategy includes early engagement with FDA through pre-submission meetings and participation in standards development organizations to shape emerging regulatory frameworks for novel bioelectronic technologies[7].
Strengths: World-class materials science and engineering capabilities enabling novel biocompatible electronic systems; strong intellectual property portfolio in bioelectronic interfaces; established relationships with regulatory science experts and agencies. Weaknesses: Challenges in translating academic research to commercial applications with full regulatory compliance; longer development timelines due to novel material characterization requirements; potential gaps between research innovations and established regulatory pathways.
Duke University
Technical Solution: Duke University has established a comprehensive approach to bioelectronic interface development that navigates complex regulatory landscapes across research and clinical applications. Their research teams have developed neural interfaces that comply with both FDA regulations and international standards while advancing novel technologies. Duke's bioelectronic platforms incorporate design controls aligned with ISO 13485 quality management systems and implement risk management strategies following ISO 14971[3]. Their neural interface technologies address biocompatibility requirements through innovative materials and coatings that meet ISO 10993 standards, particularly for long-term implantable devices. Duke researchers have pioneered closed-loop neural interface systems with sophisticated signal processing algorithms that comply with IEC 62304 software lifecycle requirements while addressing FDA guidance on artificial intelligence/machine learning in medical devices[1]. The university has established collaborative frameworks with regulatory bodies to develop appropriate testing methodologies for novel bioelectronic interfaces, particularly addressing safety concerns related to electrical stimulation parameters and tissue damage thresholds[6].
Strengths: Strong interdisciplinary research capabilities combining neuroscience, engineering, and regulatory expertise; established relationships with regulatory agencies facilitating novel approval pathways; access to clinical testing environments. Weaknesses: Academic research timelines sometimes misaligned with regulatory approval processes; challenges in scaling laboratory prototypes to commercial manufacturing standards; funding constraints compared to industry players.
Critical Standards Analysis for Biomedical Device Interfaces
Organic transistor-based system for electrophysiological monitoring of cells and method for the monitoring of the cells
PatentActiveUS20180031520A1
Innovation
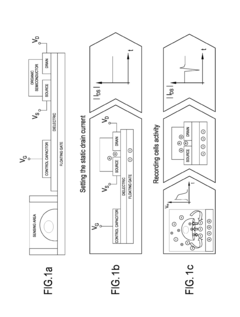
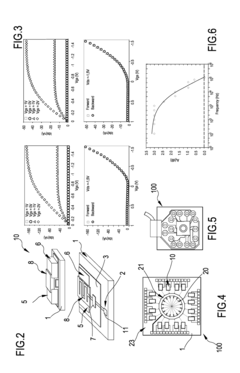
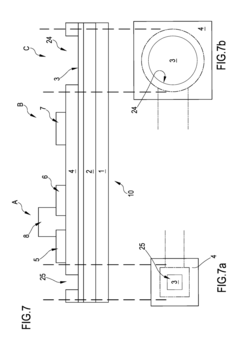
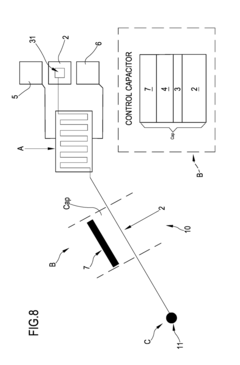
- A system comprising a plurality of organic thin film transistors with a floating gate electrode, source and drain electrodes, and an insulating layer, operated at low voltages (0.5 V to 2 V) to detect dynamic charge variations in the frequency range of cell electrical activity (1 Hz to 1000 Hz) without an external reference electrode, using a biocompatible sensing area with apertures to expose floating gates to cells, allowing for spatial mapping of cell activity.
Bioelectric potential input interface system, bioelectric potential input sensor apparatus, bioelectric potential inputting method, and program for same
PatentWO2014208074A1
Innovation
- A biopotential input interface system featuring a sensor device with expandable electrode hollow bodies, a measurement hollow body, and a housing hollow body connected in a ring, allowing for adjustable electrode spacing to accommodate different forearm sizes, and a biopotential measurement circuit connected via conductive wiring, enabling precise biopotential measurement and action detection.
Safety and Biocompatibility Requirements
Bioelectronic interfaces must adhere to stringent safety and biocompatibility requirements to ensure patient protection and regulatory compliance. The ISO 10993 series serves as the cornerstone for biocompatibility evaluation, with ISO 10993-1 providing a framework for biological assessment of medical devices. These standards mandate comprehensive testing for cytotoxicity, sensitization, irritation, systemic toxicity, and genotoxicity—all critical for devices in direct contact with human tissue.
For implantable bioelectronic interfaces, additional requirements apply under ISO 14708, which specifically addresses active implantable medical devices. This standard establishes parameters for electrical safety, electromagnetic compatibility, and long-term stability of materials in the physiological environment. The FDA's guidance document on Implanted Brain-Computer Interface Devices further refines these requirements for neural interfaces, emphasizing the need for robust hermetic sealing and corrosion resistance.
Material selection represents a critical aspect of biocompatibility compliance. Commonly used materials include medical-grade silicones, platinum-iridium alloys, and titanium, all of which demonstrate minimal inflammatory response and tissue reactivity. Recent advances in biomaterials have introduced novel coatings such as PEDOT:PSS and parylene-C, which enhance both electrical performance and biocompatibility profiles.
Sterilization validation constitutes another essential requirement, with bioelectronic interfaces typically subjected to ethylene oxide, gamma radiation, or electron beam sterilization methods as specified in ISO 11137 and ISO 11135. The chosen sterilization method must not compromise device functionality or material integrity while ensuring complete microbial elimination.
Long-term safety monitoring protocols are increasingly emphasized by regulatory bodies, with the FDA requiring post-market surveillance plans for implantable electronic devices. These plans must include strategies for monitoring device degradation, tissue response, and potential migration over extended periods—often exceeding 5-10 years for permanent implants.
Emerging regulations are beginning to address novel bioelectronic interface technologies, including flexible electronics and biodegradable components. The IEC 60601-2-76 standard, currently under development, will specifically target wearable electromedical devices, while the ASTM F3407 standard addresses absorbable materials in medical applications. These evolving frameworks reflect the rapid innovation in bioelectronic interfaces and the regulatory community's efforts to maintain appropriate safety oversight.
For implantable bioelectronic interfaces, additional requirements apply under ISO 14708, which specifically addresses active implantable medical devices. This standard establishes parameters for electrical safety, electromagnetic compatibility, and long-term stability of materials in the physiological environment. The FDA's guidance document on Implanted Brain-Computer Interface Devices further refines these requirements for neural interfaces, emphasizing the need for robust hermetic sealing and corrosion resistance.
Material selection represents a critical aspect of biocompatibility compliance. Commonly used materials include medical-grade silicones, platinum-iridium alloys, and titanium, all of which demonstrate minimal inflammatory response and tissue reactivity. Recent advances in biomaterials have introduced novel coatings such as PEDOT:PSS and parylene-C, which enhance both electrical performance and biocompatibility profiles.
Sterilization validation constitutes another essential requirement, with bioelectronic interfaces typically subjected to ethylene oxide, gamma radiation, or electron beam sterilization methods as specified in ISO 11137 and ISO 11135. The chosen sterilization method must not compromise device functionality or material integrity while ensuring complete microbial elimination.
Long-term safety monitoring protocols are increasingly emphasized by regulatory bodies, with the FDA requiring post-market surveillance plans for implantable electronic devices. These plans must include strategies for monitoring device degradation, tissue response, and potential migration over extended periods—often exceeding 5-10 years for permanent implants.
Emerging regulations are beginning to address novel bioelectronic interface technologies, including flexible electronics and biodegradable components. The IEC 60601-2-76 standard, currently under development, will specifically target wearable electromedical devices, while the ASTM F3407 standard addresses absorbable materials in medical applications. These evolving frameworks reflect the rapid innovation in bioelectronic interfaces and the regulatory community's efforts to maintain appropriate safety oversight.
Cross-Border Regulatory Harmonization Efforts
The global landscape of bioelectronic interface regulations presents significant challenges for developers seeking to navigate diverse regulatory frameworks across different countries and regions. Recognizing this complexity, several international initiatives have emerged to harmonize regulatory approaches, facilitating more efficient development and market access for innovative bioelectronic technologies.
The International Medical Device Regulators Forum (IMDRF) has established working groups specifically focused on software as a medical device (SaMD) and cybersecurity standards, which directly impact bioelectronic interfaces. Their efforts have resulted in harmonized definitions and risk classification frameworks that are increasingly being adopted by regulatory bodies worldwide, including the FDA, European Medicines Agency, and Japan's Pharmaceuticals and Medical Devices Agency.
The Medical Device Single Audit Program (MDSAP) represents another significant harmonization effort, allowing manufacturers to undergo a single regulatory audit that satisfies requirements across multiple jurisdictions including the United States, Canada, Japan, Australia, and Brazil. This program has reduced redundant inspections and streamlined compliance processes for bioelectronic interface developers operating in multiple markets.
In the European context, the EU-US Mutual Recognition Agreement (MRA) for conformity assessment of medical devices has facilitated trade while maintaining safety standards. This agreement enables recognition of quality system inspections conducted by either party, reducing duplicative regulatory burdens for bioelectronic interface manufacturers seeking transatlantic market access.
The Asia-Pacific Economic Cooperation (APEC) Life Sciences Innovation Forum has established the Regulatory Harmonization Steering Committee, which works to align regulatory approaches across the Asia-Pacific region. Their Strategic Framework for regulatory convergence has particular relevance for emerging bioelectronic technologies, as it addresses novel product categories that may not fit neatly into existing regulatory classifications.
Despite these positive developments, significant challenges remain. Regulatory harmonization efforts often struggle to keep pace with rapid technological innovation in the bioelectronic interface field. Cultural differences in risk perception and varying societal values regarding privacy, data ownership, and human enhancement continue to create divergent regulatory approaches across borders.
Looking forward, the World Health Organization's Global Model Regulatory Framework for Medical Devices offers promising pathways for further harmonization, particularly for low and middle-income countries developing their regulatory systems. This framework could help establish consistent global standards for bioelectronic interfaces while allowing appropriate flexibility for different healthcare contexts and technological capabilities.
The International Medical Device Regulators Forum (IMDRF) has established working groups specifically focused on software as a medical device (SaMD) and cybersecurity standards, which directly impact bioelectronic interfaces. Their efforts have resulted in harmonized definitions and risk classification frameworks that are increasingly being adopted by regulatory bodies worldwide, including the FDA, European Medicines Agency, and Japan's Pharmaceuticals and Medical Devices Agency.
The Medical Device Single Audit Program (MDSAP) represents another significant harmonization effort, allowing manufacturers to undergo a single regulatory audit that satisfies requirements across multiple jurisdictions including the United States, Canada, Japan, Australia, and Brazil. This program has reduced redundant inspections and streamlined compliance processes for bioelectronic interface developers operating in multiple markets.
In the European context, the EU-US Mutual Recognition Agreement (MRA) for conformity assessment of medical devices has facilitated trade while maintaining safety standards. This agreement enables recognition of quality system inspections conducted by either party, reducing duplicative regulatory burdens for bioelectronic interface manufacturers seeking transatlantic market access.
The Asia-Pacific Economic Cooperation (APEC) Life Sciences Innovation Forum has established the Regulatory Harmonization Steering Committee, which works to align regulatory approaches across the Asia-Pacific region. Their Strategic Framework for regulatory convergence has particular relevance for emerging bioelectronic technologies, as it addresses novel product categories that may not fit neatly into existing regulatory classifications.
Despite these positive developments, significant challenges remain. Regulatory harmonization efforts often struggle to keep pace with rapid technological innovation in the bioelectronic interface field. Cultural differences in risk perception and varying societal values regarding privacy, data ownership, and human enhancement continue to create divergent regulatory approaches across borders.
Looking forward, the World Health Organization's Global Model Regulatory Framework for Medical Devices offers promising pathways for further harmonization, particularly for low and middle-income countries developing their regulatory systems. This framework could help establish consistent global standards for bioelectronic interfaces while allowing appropriate flexibility for different healthcare contexts and technological capabilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!