Standards Governing the Use of Bioelectronic Interfaces in Medical Robotics
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Standards Evolution and Objectives
Bioelectronic interfaces in medical robotics have evolved significantly over the past three decades, transitioning from rudimentary signal acquisition systems to sophisticated bidirectional communication platforms between biological tissues and electronic devices. The initial development phase in the 1990s focused primarily on basic neural recording capabilities with limited channels and significant signal noise. These early interfaces were predominantly used in laboratory settings with minimal clinical applications due to biocompatibility issues and limited functionality.
The early 2000s marked a pivotal shift with the introduction of the first FDA-approved neural interface systems, establishing preliminary regulatory frameworks. During this period, standards began emerging from organizations such as IEEE, ASTM International, and ISO, addressing fundamental aspects of biocompatibility, electrical safety, and basic performance requirements. These standards, however, remained fragmented across different regulatory domains.
By the 2010s, technological advancements enabled higher channel counts, improved signal resolution, and enhanced biocompatibility through novel materials like flexible electronics and nanomaterials. This period saw the convergence of multiple technical domains, necessitating more comprehensive standardization efforts. The IEC 60601 series expanded to address specific concerns related to bioelectronic medical devices, while ISO 14708 provided guidelines for implantable neurostimulators.
Current technological evolution focuses on wireless capabilities, miniaturization, and long-term stability of bioelectronic interfaces. The integration of AI for signal processing and adaptive control has introduced new dimensions requiring standardization, particularly regarding algorithm validation and performance metrics. Recent standards development has begun addressing cybersecurity concerns, data integrity, and interoperability between different medical robotic systems utilizing bioelectronic interfaces.
The primary objectives of bioelectronic interface standardization in medical robotics encompass several critical dimensions. Safety remains paramount, with standards aiming to minimize risks associated with tissue damage, infection, electrical hazards, and long-term biocompatibility. Performance standardization seeks to establish consistent metrics for signal quality, resolution, latency, and reliability across different systems and manufacturers.
Interoperability standards are increasingly crucial as medical robotic ecosystems become more complex, requiring seamless communication between bioelectronic interfaces and various robotic components. Ethical considerations have also emerged as standardization objectives, particularly regarding data privacy, informed consent for neural data collection, and appropriate use limitations.
Looking forward, standardization efforts aim to anticipate technological advancements such as bidirectional closed-loop systems, brain-computer interfaces for robotic control, and increasingly autonomous medical robotic systems guided by bioelectronic signals. The ultimate goal is creating a unified regulatory framework that ensures safety and efficacy while enabling innovation in this rapidly evolving technological domain.
The early 2000s marked a pivotal shift with the introduction of the first FDA-approved neural interface systems, establishing preliminary regulatory frameworks. During this period, standards began emerging from organizations such as IEEE, ASTM International, and ISO, addressing fundamental aspects of biocompatibility, electrical safety, and basic performance requirements. These standards, however, remained fragmented across different regulatory domains.
By the 2010s, technological advancements enabled higher channel counts, improved signal resolution, and enhanced biocompatibility through novel materials like flexible electronics and nanomaterials. This period saw the convergence of multiple technical domains, necessitating more comprehensive standardization efforts. The IEC 60601 series expanded to address specific concerns related to bioelectronic medical devices, while ISO 14708 provided guidelines for implantable neurostimulators.
Current technological evolution focuses on wireless capabilities, miniaturization, and long-term stability of bioelectronic interfaces. The integration of AI for signal processing and adaptive control has introduced new dimensions requiring standardization, particularly regarding algorithm validation and performance metrics. Recent standards development has begun addressing cybersecurity concerns, data integrity, and interoperability between different medical robotic systems utilizing bioelectronic interfaces.
The primary objectives of bioelectronic interface standardization in medical robotics encompass several critical dimensions. Safety remains paramount, with standards aiming to minimize risks associated with tissue damage, infection, electrical hazards, and long-term biocompatibility. Performance standardization seeks to establish consistent metrics for signal quality, resolution, latency, and reliability across different systems and manufacturers.
Interoperability standards are increasingly crucial as medical robotic ecosystems become more complex, requiring seamless communication between bioelectronic interfaces and various robotic components. Ethical considerations have also emerged as standardization objectives, particularly regarding data privacy, informed consent for neural data collection, and appropriate use limitations.
Looking forward, standardization efforts aim to anticipate technological advancements such as bidirectional closed-loop systems, brain-computer interfaces for robotic control, and increasingly autonomous medical robotic systems guided by bioelectronic signals. The ultimate goal is creating a unified regulatory framework that ensures safety and efficacy while enabling innovation in this rapidly evolving technological domain.
Market Demand Analysis for Medical Robotic Interfaces
The global market for bioelectronic interfaces in medical robotics has experienced significant growth in recent years, driven by increasing demand for minimally invasive surgical procedures and precision medicine. Current market valuations indicate that the medical robotics sector reached approximately 16.1 billion USD in 2022, with bioelectronic interfaces representing a rapidly expanding segment projected to grow at a compound annual growth rate of 15.7% through 2030.
Healthcare providers worldwide are increasingly adopting robotic systems equipped with advanced bioelectronic interfaces to enhance surgical outcomes, reduce recovery times, and minimize complications. This trend is particularly evident in neurosurgery, orthopedics, and cardiovascular interventions, where precision and stability are paramount.
Patient demographics are also influencing market demand, with aging populations in developed economies requiring more surgical interventions. The World Health Organization reports that by 2030, one in six people globally will be aged 60 or over, creating sustained demand for advanced medical technologies that can address age-related conditions with greater efficacy and reduced trauma.
Regulatory environments across major markets are evolving to accommodate these technologies, with the FDA in the United States establishing dedicated pathways for bioelectronic medical devices. Similar frameworks are emerging in the European Union under the Medical Device Regulation and in Asian markets, particularly Japan and South Korea, which are investing heavily in healthcare robotics.
Hospital administrators and healthcare systems are increasingly recognizing the return on investment from robotic systems with advanced interfaces, despite high initial capital expenditures. Cost-benefit analyses demonstrate that these systems can reduce overall treatment costs by shortening hospital stays, decreasing complication rates, and improving procedural efficiency.
Consumer awareness and acceptance of robotic-assisted procedures have grown substantially, with patients often specifically requesting these advanced treatment options. This consumer-driven demand is compelling healthcare providers to adopt these technologies to remain competitive in their markets.
Emerging economies represent significant growth opportunities, with countries like China, India, and Brazil rapidly expanding their healthcare infrastructure and increasingly investing in advanced medical technologies. These markets are expected to contribute substantially to global demand growth over the next decade.
The COVID-19 pandemic has accelerated certain market trends, particularly the adoption of remote surgical capabilities and autonomous systems that reduce the need for physical proximity between healthcare providers and patients. This has created new demand vectors for bioelectronic interfaces that enable telepresence and remote operation capabilities in medical robotics.
Healthcare providers worldwide are increasingly adopting robotic systems equipped with advanced bioelectronic interfaces to enhance surgical outcomes, reduce recovery times, and minimize complications. This trend is particularly evident in neurosurgery, orthopedics, and cardiovascular interventions, where precision and stability are paramount.
Patient demographics are also influencing market demand, with aging populations in developed economies requiring more surgical interventions. The World Health Organization reports that by 2030, one in six people globally will be aged 60 or over, creating sustained demand for advanced medical technologies that can address age-related conditions with greater efficacy and reduced trauma.
Regulatory environments across major markets are evolving to accommodate these technologies, with the FDA in the United States establishing dedicated pathways for bioelectronic medical devices. Similar frameworks are emerging in the European Union under the Medical Device Regulation and in Asian markets, particularly Japan and South Korea, which are investing heavily in healthcare robotics.
Hospital administrators and healthcare systems are increasingly recognizing the return on investment from robotic systems with advanced interfaces, despite high initial capital expenditures. Cost-benefit analyses demonstrate that these systems can reduce overall treatment costs by shortening hospital stays, decreasing complication rates, and improving procedural efficiency.
Consumer awareness and acceptance of robotic-assisted procedures have grown substantially, with patients often specifically requesting these advanced treatment options. This consumer-driven demand is compelling healthcare providers to adopt these technologies to remain competitive in their markets.
Emerging economies represent significant growth opportunities, with countries like China, India, and Brazil rapidly expanding their healthcare infrastructure and increasingly investing in advanced medical technologies. These markets are expected to contribute substantially to global demand growth over the next decade.
The COVID-19 pandemic has accelerated certain market trends, particularly the adoption of remote surgical capabilities and autonomous systems that reduce the need for physical proximity between healthcare providers and patients. This has created new demand vectors for bioelectronic interfaces that enable telepresence and remote operation capabilities in medical robotics.
Current Bioelectronic Interface Challenges in Medical Robotics
The integration of bioelectronic interfaces with medical robotics represents a frontier technology with immense potential for revolutionizing healthcare delivery. However, this integration faces significant technical challenges that must be addressed before widespread clinical adoption becomes feasible. Current bioelectronic interfaces suffer from limited signal quality and reliability, particularly in long-term implantation scenarios where signal degradation occurs due to tissue reactions, electrode corrosion, and mechanical stress.
Signal processing remains a critical bottleneck, with existing algorithms struggling to effectively filter noise from meaningful neural signals in real-time applications. This challenge is particularly pronounced in dynamic clinical environments where electromagnetic interference from other medical equipment can compromise signal integrity. Additionally, the translation of detected neural signals into precise robotic movements requires sophisticated decoding algorithms that can adapt to the variability in neural patterns across different patients and over time within the same patient.
Biocompatibility presents another significant hurdle, as materials used in bioelectronic interfaces must simultaneously maintain electrical conductivity while minimizing foreign body responses. Current electrode materials often trigger inflammatory responses that lead to encapsulation, reducing signal quality over time and potentially causing tissue damage. The development of novel biomaterials that can seamlessly integrate with biological tissues while maintaining stable electrical properties remains an active area of research.
Power management constitutes a fundamental challenge for implantable bioelectronic interfaces. Current battery technologies are limited in their capacity and lifespan, necessitating periodic surgical interventions for replacement. Wireless power transmission approaches show promise but face efficiency limitations and potential tissue heating concerns. Energy harvesting from biological sources represents an emerging alternative but has yet to demonstrate sufficient power generation for complex robotic control applications.
Miniaturization of bioelectronic components presents significant engineering challenges, particularly in achieving high-density electrode arrays while maintaining signal integrity. Current fabrication techniques struggle to produce ultra-small components with consistent electrical properties and mechanical durability. The integration of sensing, processing, and communication components into compact, implantable systems requires further advances in microfabrication and packaging technologies.
Standardization across bioelectronic interface technologies remains inadequate, with various research groups and companies developing proprietary systems with limited interoperability. This fragmentation impedes clinical translation and comparative evaluation of different approaches. The development of consensus standards for performance metrics, safety evaluation, and interoperability protocols would accelerate innovation and clinical adoption of bioelectronic interfaces in medical robotics.
Signal processing remains a critical bottleneck, with existing algorithms struggling to effectively filter noise from meaningful neural signals in real-time applications. This challenge is particularly pronounced in dynamic clinical environments where electromagnetic interference from other medical equipment can compromise signal integrity. Additionally, the translation of detected neural signals into precise robotic movements requires sophisticated decoding algorithms that can adapt to the variability in neural patterns across different patients and over time within the same patient.
Biocompatibility presents another significant hurdle, as materials used in bioelectronic interfaces must simultaneously maintain electrical conductivity while minimizing foreign body responses. Current electrode materials often trigger inflammatory responses that lead to encapsulation, reducing signal quality over time and potentially causing tissue damage. The development of novel biomaterials that can seamlessly integrate with biological tissues while maintaining stable electrical properties remains an active area of research.
Power management constitutes a fundamental challenge for implantable bioelectronic interfaces. Current battery technologies are limited in their capacity and lifespan, necessitating periodic surgical interventions for replacement. Wireless power transmission approaches show promise but face efficiency limitations and potential tissue heating concerns. Energy harvesting from biological sources represents an emerging alternative but has yet to demonstrate sufficient power generation for complex robotic control applications.
Miniaturization of bioelectronic components presents significant engineering challenges, particularly in achieving high-density electrode arrays while maintaining signal integrity. Current fabrication techniques struggle to produce ultra-small components with consistent electrical properties and mechanical durability. The integration of sensing, processing, and communication components into compact, implantable systems requires further advances in microfabrication and packaging technologies.
Standardization across bioelectronic interface technologies remains inadequate, with various research groups and companies developing proprietary systems with limited interoperability. This fragmentation impedes clinical translation and comparative evaluation of different approaches. The development of consensus standards for performance metrics, safety evaluation, and interoperability protocols would accelerate innovation and clinical adoption of bioelectronic interfaces in medical robotics.
Current Standardization Frameworks for Bioelectronic Interfaces
01 Neural interfaces for bioelectronic applications
Neural interfaces are a key component in bioelectronic systems that establish connections between electronic devices and the nervous system. These interfaces enable bidirectional communication, allowing for both recording of neural signals and stimulation of neural tissues. Advanced materials and fabrication techniques are employed to create flexible, biocompatible interfaces that minimize tissue damage and immune response while maintaining long-term functionality. These technologies have applications in neuroprosthetics, brain-computer interfaces, and therapeutic devices for neurological disorders.- Neural interfaces for bioelectronic applications: Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neurons, or both, enabling applications in neural prosthetics, brain-computer interfaces, and treatment of neurological disorders. Advanced materials and fabrication techniques are used to create biocompatible electrodes that can effectively interface with neural tissue while minimizing tissue damage and inflammatory responses.
- Flexible and stretchable bioelectronic interfaces: Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic and curved surfaces of biological tissues. These interfaces incorporate elastic materials, serpentine structures, or mesh designs to achieve mechanical compliance while maintaining electronic functionality. Such flexibility reduces mechanical mismatch between rigid electronics and soft tissues, improving long-term biocompatibility and enabling applications in wearable health monitoring, implantable devices, and soft robotics.
- Biomolecular interfaces for biosensing and diagnostics: Biomolecular interfaces integrate biological recognition elements with electronic transduction systems to create highly specific and sensitive biosensors. These interfaces may incorporate enzymes, antibodies, nucleic acids, or other biomolecules that selectively interact with target analytes. The resulting electronic signals can be processed to detect biomarkers, pathogens, or environmental contaminants at low concentrations, enabling applications in medical diagnostics, environmental monitoring, and food safety.
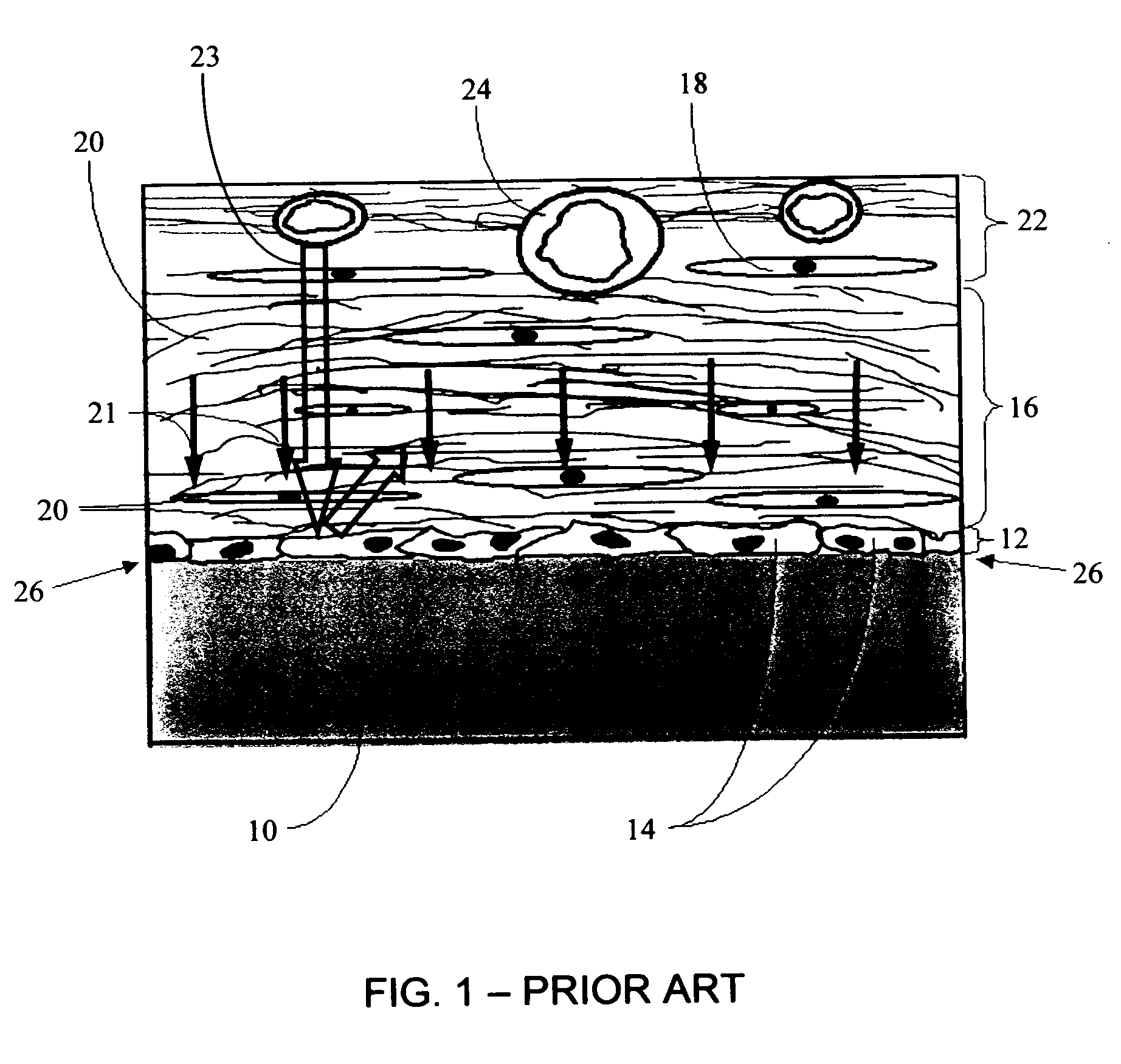
- Implantable bioelectronic devices for therapeutic applications: Implantable bioelectronic devices are designed to be placed within the body to monitor physiological parameters or deliver therapeutic interventions. These devices incorporate biocompatible materials, power management systems, and wireless communication capabilities to ensure long-term functionality within the biological environment. Applications include cardiac pacemakers, neurostimulators for pain management, drug delivery systems, and implantable sensors for continuous health monitoring.
- Nanomaterial-based bioelectronic interfaces: Nanomaterial-based bioelectronic interfaces leverage the unique properties of nanomaterials such as carbon nanotubes, graphene, quantum dots, and metal nanoparticles to enhance the performance of bioelectronic devices. These materials offer advantages including high surface-to-volume ratio, exceptional electrical conductivity, and tunable surface chemistry. Nanomaterial interfaces enable improved signal transduction, enhanced sensitivity, and miniaturization of bioelectronic devices for applications ranging from neural recording to molecular detection.
02 Implantable bioelectronic devices
Implantable bioelectronic devices are designed to integrate with biological tissues for monitoring, diagnostic, or therapeutic purposes. These devices incorporate biocompatible materials, miniaturized electronics, and power management systems to ensure long-term functionality within the body. Key considerations include hermeticity, biofouling prevention, and wireless communication capabilities. Applications include cardiac pacemakers, neural stimulators, drug delivery systems, and continuous physiological monitoring platforms that can provide real-time health data and therapeutic interventions.Expand Specific Solutions03 Biosensing and bioelectronic detection systems
Biosensing and bioelectronic detection systems utilize the interface between biological components and electronic transducers to detect and quantify biological analytes. These systems incorporate various sensing modalities including electrochemical, optical, and mechanical transduction mechanisms. Advanced materials such as nanomaterials, conductive polymers, and functionalized surfaces enhance sensitivity and selectivity. Applications include point-of-care diagnostics, continuous health monitoring, environmental sensing, and biomarker detection for early disease diagnosis and personalized medicine approaches.Expand Specific Solutions04 Flexible and wearable bioelectronic interfaces
Flexible and wearable bioelectronic interfaces are designed to conform to the dynamic surfaces of biological tissues while maintaining electronic functionality. These interfaces utilize stretchable substrates, serpentine interconnects, and thin-film electronics to achieve mechanical compliance with soft tissues. Advanced manufacturing techniques such as transfer printing and direct writing enable the fabrication of complex, multi-layered devices. Applications include skin-mounted sensors, electronic textiles, epidermal electronics, and conformal neural interfaces that can monitor physiological parameters without restricting natural movement.Expand Specific Solutions05 Bioelectronic materials and fabrication methods
Advanced materials and fabrication methods are essential for creating effective bioelectronic interfaces. These include conducting polymers, hydrogels, carbon nanomaterials, and biohybrid composites that bridge the mechanical and electrical mismatch between conventional electronics and biological tissues. Novel fabrication approaches such as 3D printing, soft lithography, and directed assembly enable precise control over interface architecture at multiple scales. Biofunctionalization strategies incorporate bioactive molecules to improve biocompatibility, reduce foreign body response, and promote specific biological interactions at the electronic interface.Expand Specific Solutions
Key Industry Players in Bioelectronic Medical Robotics
The bioelectronic interfaces in medical robotics standards landscape is currently in a transitional phase, moving from early development to commercial application. The market is projected to reach significant growth as healthcare systems increasingly adopt robotic-assisted procedures. Leading research institutions like MIT, University of Michigan, and Caltech are establishing foundational technologies, while commercial players demonstrate varying levels of technical maturity. Medtronic and Boston Scientific (via Cardiac Pacemakers) have achieved advanced integration of bioelectronic interfaces in medical devices, while newer entrants like Saluda Medical and DiFusion are developing specialized applications. Technology companies including Google, Apple, and Meta are leveraging their AI and sensing capabilities to enter this space, indicating cross-industry convergence and accelerating innovation in bioelectronic medical standards.
Medtronic, Inc.
Technical Solution: Medtronic has developed comprehensive bioelectronic interface systems for medical robotics that comply with IEC 60601 standards for medical electrical equipment safety. Their approach integrates closed-loop neural interfaces with adaptive algorithms that continuously monitor neural signals and adjust stimulation parameters in real-time. The company's proprietary SureStim™ technology enables precise neuromodulation with minimal tissue damage through ultra-thin flexible electrodes that conform to neural tissue. Medtronic has pioneered implantable pulse generators (IPGs) that meet ISO 14708 standards for active implantable medical devices, featuring wireless communication protocols that adhere to IEEE 802.15.6 standards for wireless body area networks. Their systems incorporate fail-safe mechanisms that comply with IEC 62304 for medical device software and IEC 62366 for usability engineering in medical devices.
Strengths: Extensive clinical validation with large patient datasets; robust regulatory compliance framework across multiple jurisdictions; advanced miniaturization capabilities for implantable devices. Weaknesses: Proprietary systems limit interoperability with other manufacturers' devices; higher implementation costs compared to competitors; relatively slower update cycles for software components due to rigorous validation requirements.
California Institute of Technology
Technical Solution: Caltech's Division of Engineering and Applied Science has pioneered groundbreaking research in bioelectronic interfaces for medical robotics, with particular emphasis on brain-machine interfaces (BMIs) and neuroprosthetics. Their approach integrates advanced materials science with computational neuroscience to develop high-density electrode arrays that achieve single-neuron recording resolution while minimizing tissue damage. Caltech researchers have developed novel flexible electronics platforms that conform to biological tissues, featuring stretchable substrates with embedded microelectrodes that maintain functionality under deformation up to 30% strain. Their neural decoding algorithms incorporate deep learning architectures that achieve decoding accuracies exceeding 90% for complex motor intentions from multichannel neural recordings. Caltech's work addresses biocompatibility challenges through innovative coatings that reduce foreign body responses and promote long-term stability of neural interfaces, with in vivo studies demonstrating stable recordings for periods exceeding 18 months. Their research complies with IEEE standards for neural interface technology while pushing boundaries in materials, fabrication techniques, and computational approaches.
Strengths: World-class interdisciplinary research combining neuroscience, materials science, and robotics; strong focus on fundamental scientific advances that enable next-generation interfaces; extensive collaboration network with clinical and industry partners. Weaknesses: Primary focus on research rather than commercialization; longer timeline to clinical implementation compared to industry players; funding dependent on grants and academic partnerships rather than commercial revenue streams.
Critical Patents and Research in Medical Interface Technology
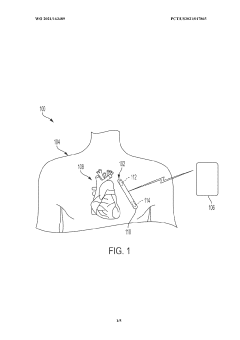
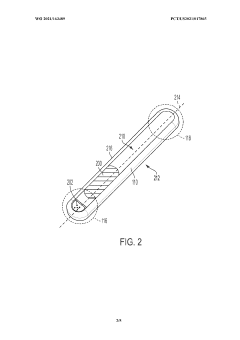
Implantable medical device having a biocompatible circuit board with embedded electrodes
PatentWO2021163489A1
Innovation
- The implementation of a biocompatible circuit board with embedded electrodes and an antenna positioned proximate to a non-conductive window, eliminating the need for a header and allowing for a larger volume within the IMD for increased electronics and power supply, while maintaining biocompatibility and efficient wireless communication.
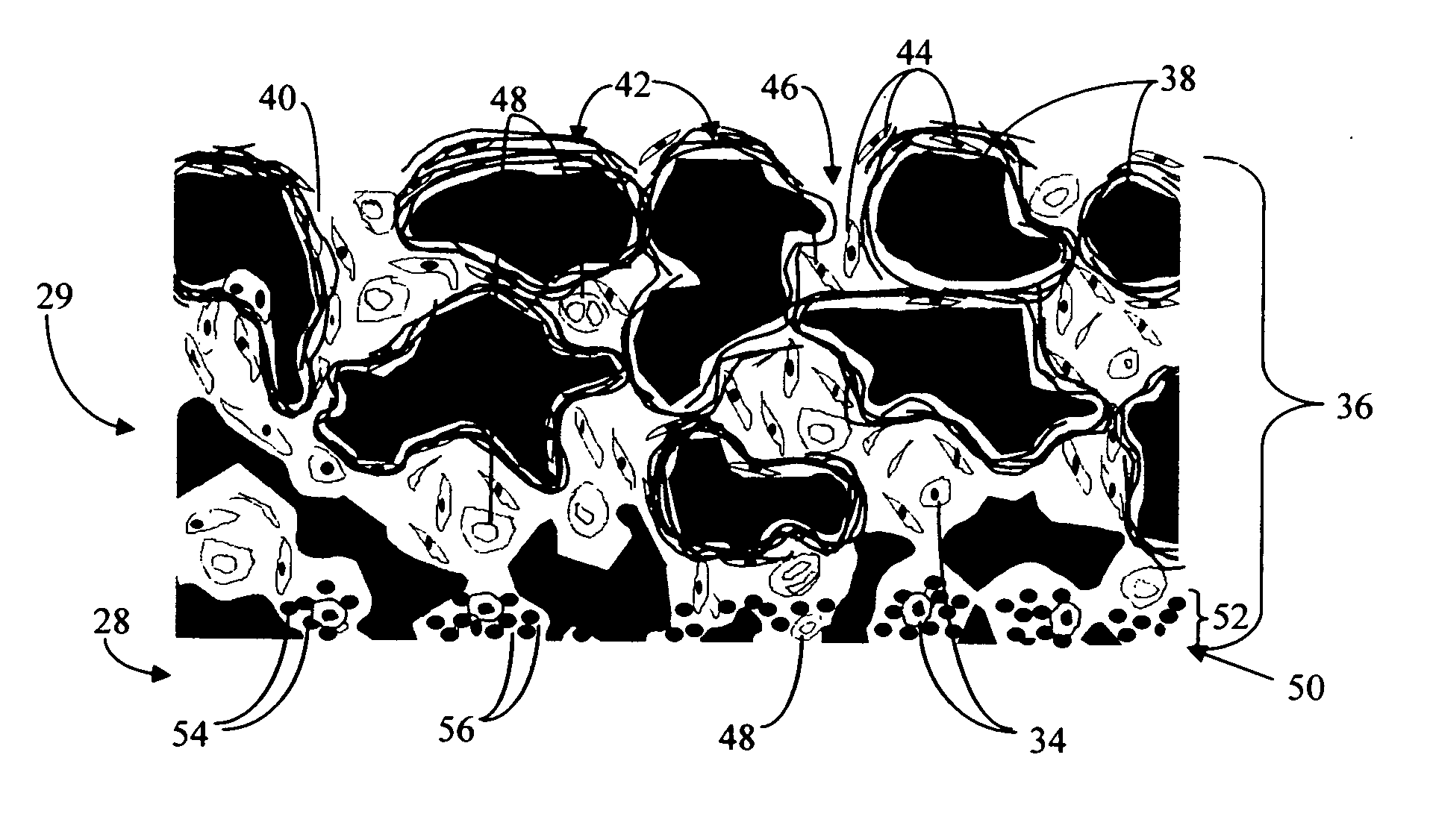
Biointerface with macro-and micro-architecture
PatentActiveUS20050251083A1
Innovation
- A biointerface membrane with a macro-architecture featuring interconnected cavities greater than 20 microns and a micro-architecture with elongated strands less than 20 microns is used to support tissue ingrowth and interfere with barrier cell layer formation, facilitating long-term analyte transport.
Regulatory Compliance and Safety Requirements
The regulatory landscape for bioelectronic interfaces in medical robotics is complex and multifaceted, requiring compliance with numerous standards across different jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies most bioelectronic medical devices under Class II or III, necessitating either 510(k) clearance or Premarket Approval (PMA). These regulatory pathways demand extensive clinical data demonstrating both safety and efficacy, with particular emphasis on biocompatibility, electrical safety, and long-term reliability.
The International Electrotechnical Commission (IEC) has established several critical standards specifically applicable to bioelectronic interfaces, including IEC 60601-1 for general safety requirements of medical electrical equipment and IEC 60601-2-X series for particular requirements based on specific applications. Additionally, ISO 13485 provides the framework for quality management systems that manufacturers must implement to ensure consistent production of safe devices.
For neural interfaces specifically, the IEEE has developed standards such as IEEE P2794 for reporting neural interface data and IEEE 1708 for wearable, cuffless blood pressure measuring devices. These standards address the unique challenges of brain-computer interfaces and neural stimulation devices, including signal quality, artifact rejection, and data interpretation protocols.
Safety requirements for bioelectronic interfaces extend beyond electrical considerations to include biocompatibility testing according to ISO 10993 series. This framework evaluates potential biological risks including cytotoxicity, sensitization, irritation, and systemic toxicity. For implantable devices, additional testing for carcinogenicity and long-term implantation effects is mandatory.
Cybersecurity has emerged as a critical safety concern for connected bioelectronic devices. The FDA's guidance on "Content of Premarket Submissions for Management of Cybersecurity in Medical Devices" outlines requirements for threat modeling, vulnerability assessment, and security risk management throughout the device lifecycle. Similarly, the EU's Medical Device Regulation (MDR) includes specific provisions for software validation and cybersecurity measures.
Risk management processes following ISO 14971 are fundamental to regulatory compliance, requiring manufacturers to identify hazards, estimate and evaluate associated risks, implement control measures, and monitor their effectiveness. For bioelectronic interfaces, particular attention must be paid to risks related to electrical stimulation parameters, tissue damage, infection, and device migration or failure.
Post-market surveillance requirements have become increasingly stringent, with regulatory bodies requiring robust systems for adverse event reporting, trend analysis, and corrective actions. The EU MDR has significantly expanded these requirements, mandating periodic safety update reports and more comprehensive post-market clinical follow-up for high-risk devices.
The International Electrotechnical Commission (IEC) has established several critical standards specifically applicable to bioelectronic interfaces, including IEC 60601-1 for general safety requirements of medical electrical equipment and IEC 60601-2-X series for particular requirements based on specific applications. Additionally, ISO 13485 provides the framework for quality management systems that manufacturers must implement to ensure consistent production of safe devices.
For neural interfaces specifically, the IEEE has developed standards such as IEEE P2794 for reporting neural interface data and IEEE 1708 for wearable, cuffless blood pressure measuring devices. These standards address the unique challenges of brain-computer interfaces and neural stimulation devices, including signal quality, artifact rejection, and data interpretation protocols.
Safety requirements for bioelectronic interfaces extend beyond electrical considerations to include biocompatibility testing according to ISO 10993 series. This framework evaluates potential biological risks including cytotoxicity, sensitization, irritation, and systemic toxicity. For implantable devices, additional testing for carcinogenicity and long-term implantation effects is mandatory.
Cybersecurity has emerged as a critical safety concern for connected bioelectronic devices. The FDA's guidance on "Content of Premarket Submissions for Management of Cybersecurity in Medical Devices" outlines requirements for threat modeling, vulnerability assessment, and security risk management throughout the device lifecycle. Similarly, the EU's Medical Device Regulation (MDR) includes specific provisions for software validation and cybersecurity measures.
Risk management processes following ISO 14971 are fundamental to regulatory compliance, requiring manufacturers to identify hazards, estimate and evaluate associated risks, implement control measures, and monitor their effectiveness. For bioelectronic interfaces, particular attention must be paid to risks related to electrical stimulation parameters, tissue damage, infection, and device migration or failure.
Post-market surveillance requirements have become increasingly stringent, with regulatory bodies requiring robust systems for adverse event reporting, trend analysis, and corrective actions. The EU MDR has significantly expanded these requirements, mandating periodic safety update reports and more comprehensive post-market clinical follow-up for high-risk devices.
Ethical and Patient Privacy Considerations
The integration of bioelectronic interfaces in medical robotics raises significant ethical and patient privacy considerations that must be addressed through comprehensive standards and frameworks. Patient autonomy represents a fundamental ethical principle that requires patients to provide informed consent before any bioelectronic interface is implemented. This consent process must include clear explanations of data collection practices, potential risks, and the specific purposes for which the collected data will be used. Standards must ensure that patients maintain the right to withdraw consent at any time without negative consequences to their care quality.
Data security in bioelectronic medical systems presents unique challenges due to the sensitive nature of the information collected. These devices often capture intimate physiological and neurological data that could reveal highly personal information beyond what is necessary for treatment. Standards must mandate robust encryption protocols, secure authentication mechanisms, and comprehensive audit trails to protect against unauthorized access or data breaches. Additionally, regulations should specify data minimization principles, ensuring that only essential information is collected and retained for the minimum necessary period.
The potential for algorithmic bias in bioelectronic interfaces represents another critical ethical concern. When these systems incorporate machine learning or artificial intelligence components, they may perpetuate or amplify existing healthcare disparities if trained on non-representative data sets. Standards should require rigorous validation of algorithms across diverse patient populations and mandate transparency regarding the limitations of the technology. Regular auditing of system outputs for potential discriminatory patterns must be established as standard practice.
Long-term monitoring capabilities of bioelectronic interfaces raise questions about continuous surveillance and its psychological impact on patients. Standards must address the potential for these technologies to create a sense of constant medical observation, which may induce anxiety or behavioral modifications in patients aware of ongoing monitoring. Guidelines should establish clear boundaries regarding monitoring duration, frequency, and scope, with particular attention to preserving patient dignity and psychological well-being.
Cross-border data transfer considerations become increasingly relevant as medical robotics systems often operate within global healthcare networks. Standards must address the varying international regulations regarding medical data protection and establish clear protocols for secure and compliant data sharing across jurisdictions. This includes alignment with frameworks such as GDPR in Europe, HIPAA in the United States, and emerging regulations in other regions to ensure consistent protection of patient information regardless of geographic location.
Data security in bioelectronic medical systems presents unique challenges due to the sensitive nature of the information collected. These devices often capture intimate physiological and neurological data that could reveal highly personal information beyond what is necessary for treatment. Standards must mandate robust encryption protocols, secure authentication mechanisms, and comprehensive audit trails to protect against unauthorized access or data breaches. Additionally, regulations should specify data minimization principles, ensuring that only essential information is collected and retained for the minimum necessary period.
The potential for algorithmic bias in bioelectronic interfaces represents another critical ethical concern. When these systems incorporate machine learning or artificial intelligence components, they may perpetuate or amplify existing healthcare disparities if trained on non-representative data sets. Standards should require rigorous validation of algorithms across diverse patient populations and mandate transparency regarding the limitations of the technology. Regular auditing of system outputs for potential discriminatory patterns must be established as standard practice.
Long-term monitoring capabilities of bioelectronic interfaces raise questions about continuous surveillance and its psychological impact on patients. Standards must address the potential for these technologies to create a sense of constant medical observation, which may induce anxiety or behavioral modifications in patients aware of ongoing monitoring. Guidelines should establish clear boundaries regarding monitoring duration, frequency, and scope, with particular attention to preserving patient dignity and psychological well-being.
Cross-border data transfer considerations become increasingly relevant as medical robotics systems often operate within global healthcare networks. Standards must address the varying international regulations regarding medical data protection and establish clear protocols for secure and compliant data sharing across jurisdictions. This includes alignment with frameworks such as GDPR in Europe, HIPAA in the United States, and emerging regulations in other regions to ensure consistent protection of patient information regardless of geographic location.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!