The Influence of Ambient Conditions on Bioelectronic Interface Stability
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Stability Background and Objectives
Bioelectronic interfaces represent a revolutionary frontier in medical technology, bridging the gap between electronic devices and biological systems. These interfaces have evolved significantly over the past three decades, transitioning from rudimentary electrode-based systems to sophisticated, multifunctional platforms capable of bidirectional communication with living tissues. The trajectory of development has been characterized by continuous miniaturization, enhanced biocompatibility, and improved signal fidelity.
The stability of bioelectronic interfaces under varying ambient conditions remains a critical challenge that impedes their widespread clinical adoption. Historical data indicates that early interfaces suffered from rapid performance degradation when exposed to physiological environments, with functional lifespans often limited to weeks or months. Recent technological advances have extended this timeframe, yet long-term stability continues to be an elusive goal.
Temperature fluctuations represent a primary destabilizing factor, as they can alter material properties, accelerate degradation reactions, and modify the behavior of biological components at the interface. Similarly, humidity variations affect the hydration state of biomaterials and can promote corrosion of electronic components. Mechanical stresses, whether from tissue movement or external forces, frequently compromise interface integrity through delamination or fracture.
The chemical complexity of biological environments presents additional challenges, with protein adsorption, enzymatic degradation, and immune responses all contributing to interface failure. pH variations, particularly in implantable applications, can accelerate material degradation and alter electronic performance. Oxidative stress, ubiquitous in biological systems, attacks vulnerable materials and compromises electronic functionality.
Our technical objectives focus on developing bioelectronic interfaces with enhanced resilience to ambient condition variations. Specifically, we aim to: (1) characterize the influence of temperature, humidity, mechanical stress, and chemical factors on interface stability; (2) develop predictive models for interface degradation under varying conditions; (3) design novel materials and architectures that maintain performance across a broader range of ambient parameters; and (4) establish standardized testing protocols that realistically simulate the complex, dynamic environments these interfaces must withstand.
The ultimate goal is to create bioelectronic interfaces that maintain stable performance for at least five years under physiologically relevant conditions, representing a tenfold improvement over current capabilities. This advancement would enable transformative applications in neural prosthetics, continuous health monitoring, and targeted drug delivery systems, significantly expanding the clinical utility of bioelectronic technologies.
The stability of bioelectronic interfaces under varying ambient conditions remains a critical challenge that impedes their widespread clinical adoption. Historical data indicates that early interfaces suffered from rapid performance degradation when exposed to physiological environments, with functional lifespans often limited to weeks or months. Recent technological advances have extended this timeframe, yet long-term stability continues to be an elusive goal.
Temperature fluctuations represent a primary destabilizing factor, as they can alter material properties, accelerate degradation reactions, and modify the behavior of biological components at the interface. Similarly, humidity variations affect the hydration state of biomaterials and can promote corrosion of electronic components. Mechanical stresses, whether from tissue movement or external forces, frequently compromise interface integrity through delamination or fracture.
The chemical complexity of biological environments presents additional challenges, with protein adsorption, enzymatic degradation, and immune responses all contributing to interface failure. pH variations, particularly in implantable applications, can accelerate material degradation and alter electronic performance. Oxidative stress, ubiquitous in biological systems, attacks vulnerable materials and compromises electronic functionality.
Our technical objectives focus on developing bioelectronic interfaces with enhanced resilience to ambient condition variations. Specifically, we aim to: (1) characterize the influence of temperature, humidity, mechanical stress, and chemical factors on interface stability; (2) develop predictive models for interface degradation under varying conditions; (3) design novel materials and architectures that maintain performance across a broader range of ambient parameters; and (4) establish standardized testing protocols that realistically simulate the complex, dynamic environments these interfaces must withstand.
The ultimate goal is to create bioelectronic interfaces that maintain stable performance for at least five years under physiologically relevant conditions, representing a tenfold improvement over current capabilities. This advancement would enable transformative applications in neural prosthetics, continuous health monitoring, and targeted drug delivery systems, significantly expanding the clinical utility of bioelectronic technologies.
Market Analysis for Environment-Resistant Bioelectronics
The bioelectronic interface market is experiencing significant growth, driven by increasing applications in healthcare monitoring, neural interfaces, and implantable medical devices. Current market valuations indicate the global bioelectronics sector is expanding at a compound annual growth rate of 14.2%, with environment-resistant bioelectronics representing an emerging high-value segment. This growth trajectory is expected to continue as healthcare systems worldwide seek more durable and reliable bioelectronic solutions.
Consumer demand for bioelectronic devices that maintain functionality across diverse environmental conditions is rising sharply. Healthcare providers require devices that perform consistently in hospital settings with varying humidity levels and potential exposure to bodily fluids. Similarly, wearable health monitoring systems must withstand perspiration, temperature fluctuations, and occasional water exposure while maintaining signal integrity and patient safety.
Market research reveals particular demand growth in regions with extreme climate conditions. Countries with high humidity levels in Southeast Asia show increased interest in moisture-resistant bioelectronic interfaces, while markets in arid regions prioritize dust and heat resistance. This geographical variation in requirements presents both challenges and opportunities for manufacturers developing versatile bioelectronic solutions.
The competitive landscape shows several distinct market segments emerging. Premium medical-grade bioelectronics with comprehensive environmental resistance command higher price points and target hospital and clinical settings. Meanwhile, consumer-grade wearable bioelectronics emphasize specific environmental resistances relevant to daily use, such as sweat resistance and limited water exposure protection, at more accessible price points.
Industry surveys indicate that 78% of healthcare professionals consider environmental stability a critical factor when selecting bioelectronic interfaces for clinical applications. Patient satisfaction data similarly shows that device reliability under varying conditions significantly impacts adherence rates for wearable bioelectronic monitoring systems.
Investment patterns reveal growing capital allocation toward research addressing bioelectronic stability challenges. Venture capital funding for startups focused on environment-resistant bioelectronics increased by 32% in the past year, indicating strong market confidence in this technological direction. Major medical device manufacturers are also expanding their R&D budgets specifically for improving interface stability across diverse ambient conditions.
Market forecasts suggest that bioelectronic interfaces capable of maintaining stability across multiple environmental challenges will capture premium market positioning. The ability to withstand combinations of humidity, temperature fluctuation, mechanical stress, and chemical exposure represents the highest value proposition for next-generation devices, particularly for implantable technologies where replacement involves significant medical intervention.
Consumer demand for bioelectronic devices that maintain functionality across diverse environmental conditions is rising sharply. Healthcare providers require devices that perform consistently in hospital settings with varying humidity levels and potential exposure to bodily fluids. Similarly, wearable health monitoring systems must withstand perspiration, temperature fluctuations, and occasional water exposure while maintaining signal integrity and patient safety.
Market research reveals particular demand growth in regions with extreme climate conditions. Countries with high humidity levels in Southeast Asia show increased interest in moisture-resistant bioelectronic interfaces, while markets in arid regions prioritize dust and heat resistance. This geographical variation in requirements presents both challenges and opportunities for manufacturers developing versatile bioelectronic solutions.
The competitive landscape shows several distinct market segments emerging. Premium medical-grade bioelectronics with comprehensive environmental resistance command higher price points and target hospital and clinical settings. Meanwhile, consumer-grade wearable bioelectronics emphasize specific environmental resistances relevant to daily use, such as sweat resistance and limited water exposure protection, at more accessible price points.
Industry surveys indicate that 78% of healthcare professionals consider environmental stability a critical factor when selecting bioelectronic interfaces for clinical applications. Patient satisfaction data similarly shows that device reliability under varying conditions significantly impacts adherence rates for wearable bioelectronic monitoring systems.
Investment patterns reveal growing capital allocation toward research addressing bioelectronic stability challenges. Venture capital funding for startups focused on environment-resistant bioelectronics increased by 32% in the past year, indicating strong market confidence in this technological direction. Major medical device manufacturers are also expanding their R&D budgets specifically for improving interface stability across diverse ambient conditions.
Market forecasts suggest that bioelectronic interfaces capable of maintaining stability across multiple environmental challenges will capture premium market positioning. The ability to withstand combinations of humidity, temperature fluctuation, mechanical stress, and chemical exposure represents the highest value proposition for next-generation devices, particularly for implantable technologies where replacement involves significant medical intervention.
Current Challenges in Ambient Condition Tolerance
Bioelectronic interfaces face significant challenges when exposed to varying ambient conditions, which can substantially impact their stability and performance. The primary challenge lies in maintaining consistent functionality across fluctuating environmental parameters such as temperature, humidity, and atmospheric pressure. These interfaces, which bridge biological systems with electronic components, are inherently sensitive to environmental changes due to their hybrid nature.
Temperature variations present a formidable obstacle, as they can alter the electrical properties of materials, affect reaction kinetics at the bio-interface, and induce mechanical stress through thermal expansion and contraction. Current bioelectronic systems typically operate optimally within narrow temperature ranges, with performance degradation occurring outside these boundaries. This limitation severely restricts their deployment in real-world applications where temperature control is impractical.
Humidity poses another critical challenge, particularly for implantable or wearable bioelectronic devices. Moisture can penetrate protective layers, leading to corrosion of electronic components, short circuits, and degradation of biomolecular elements. The water vapor permeability of encapsulation materials remains insufficient for long-term protection, especially in high-humidity environments or during transitions between different humidity levels.
Oxidative stress from atmospheric oxygen represents a significant threat to bioelectronic interface stability. Oxidation can compromise both electronic components and biological elements, altering surface chemistry and degrading functional groups essential for bio-recognition. Current antioxidant strategies and oxygen-barrier materials provide only temporary protection, failing to address long-term exposure effects.
Mechanical stability under varying ambient conditions constitutes another major challenge. Changes in temperature and humidity can induce swelling, contraction, or delamination at material interfaces, compromising the structural integrity of bioelectronic devices. These mechanical failures often initiate at microscopic levels before manifesting as catastrophic device failure, making early detection difficult.
The combined effect of multiple ambient factors creates complex failure modes that are difficult to predict and mitigate. For instance, the synergistic interaction between temperature fluctuations and humidity can accelerate degradation processes beyond what would be expected from either factor alone. Current testing protocols typically evaluate these factors in isolation, failing to capture real-world multifactorial environmental stresses.
Standardization of testing methodologies for ambient condition tolerance remains underdeveloped, hindering comparative analysis between different bioelectronic interfaces. The lack of universally accepted accelerated aging protocols specific to bioelectronic systems makes lifetime predictions unreliable, creating uncertainty for both developers and end-users regarding long-term performance expectations.
Temperature variations present a formidable obstacle, as they can alter the electrical properties of materials, affect reaction kinetics at the bio-interface, and induce mechanical stress through thermal expansion and contraction. Current bioelectronic systems typically operate optimally within narrow temperature ranges, with performance degradation occurring outside these boundaries. This limitation severely restricts their deployment in real-world applications where temperature control is impractical.
Humidity poses another critical challenge, particularly for implantable or wearable bioelectronic devices. Moisture can penetrate protective layers, leading to corrosion of electronic components, short circuits, and degradation of biomolecular elements. The water vapor permeability of encapsulation materials remains insufficient for long-term protection, especially in high-humidity environments or during transitions between different humidity levels.
Oxidative stress from atmospheric oxygen represents a significant threat to bioelectronic interface stability. Oxidation can compromise both electronic components and biological elements, altering surface chemistry and degrading functional groups essential for bio-recognition. Current antioxidant strategies and oxygen-barrier materials provide only temporary protection, failing to address long-term exposure effects.
Mechanical stability under varying ambient conditions constitutes another major challenge. Changes in temperature and humidity can induce swelling, contraction, or delamination at material interfaces, compromising the structural integrity of bioelectronic devices. These mechanical failures often initiate at microscopic levels before manifesting as catastrophic device failure, making early detection difficult.
The combined effect of multiple ambient factors creates complex failure modes that are difficult to predict and mitigate. For instance, the synergistic interaction between temperature fluctuations and humidity can accelerate degradation processes beyond what would be expected from either factor alone. Current testing protocols typically evaluate these factors in isolation, failing to capture real-world multifactorial environmental stresses.
Standardization of testing methodologies for ambient condition tolerance remains underdeveloped, hindering comparative analysis between different bioelectronic interfaces. The lack of universally accepted accelerated aging protocols specific to bioelectronic systems makes lifetime predictions unreliable, creating uncertainty for both developers and end-users regarding long-term performance expectations.
Existing Solutions for Environmental Stability
01 Surface modification techniques for bioelectronic interface stability
Various surface modification techniques can be employed to enhance the stability of bioelectronic interfaces. These include chemical functionalization, polymer coating, and surface patterning that improve biocompatibility and reduce biofouling. Such modifications create a more stable interface between electronic components and biological tissues, extending the functional lifetime of bioelectronic devices and ensuring consistent performance over time.- Surface modification techniques for bioelectronic interface stability: Various surface modification techniques can be employed to enhance the stability of bioelectronic interfaces. These include chemical functionalization of electrode surfaces, application of biocompatible coatings, and surface patterning to improve adhesion between biological tissues and electronic components. These modifications can reduce biofouling, prevent immune rejection, and maintain long-term functional stability of the interface.
- Encapsulation materials for improved durability: Specialized encapsulation materials can significantly enhance the durability and longevity of bioelectronic interfaces. These materials protect sensitive electronic components from bodily fluids, prevent corrosion, and maintain device functionality in biological environments. Advanced polymers, hydrogels, and composite materials with tunable properties are being developed to provide flexible yet robust protection while maintaining biocompatibility.
- Anti-fouling strategies for long-term implantable devices: Anti-fouling strategies are crucial for maintaining the stability of long-term implantable bioelectronic interfaces. These approaches include the incorporation of anti-fouling polymers, zwitterionic materials, and enzyme-based cleaning mechanisms that actively prevent protein adsorption and cellular attachment. Such strategies help maintain signal quality and extend the functional lifetime of implanted devices by preventing the formation of fibrous encapsulation.
- Self-healing and adaptive interface technologies: Self-healing and adaptive technologies represent an innovative approach to bioelectronic interface stability. These systems can autonomously repair damage, adapt to changing biological conditions, and maintain optimal performance over time. Materials with intrinsic self-healing properties, stimuli-responsive polymers, and dynamic reconfiguration capabilities enable interfaces that can respond to mechanical stress, chemical changes, or biological processes to maintain stable connections.
- Biomimetic approaches for enhanced biocompatibility: Biomimetic approaches involve designing bioelectronic interfaces that mimic natural biological structures and functions to enhance biocompatibility and stability. These include incorporating extracellular matrix components, using tissue-like mechanical properties, and developing interfaces with similar biochemical characteristics to native tissues. By closely resembling the biological environment, these interfaces reduce foreign body responses and promote more stable long-term integration with living tissues.
02 Encapsulation materials for long-term bioelectronic stability
Advanced encapsulation materials play a crucial role in protecting bioelectronic interfaces from degradation in biological environments. These materials include biocompatible polymers, ceramics, and composite structures that shield electronic components from moisture, ions, and enzymatic activity while maintaining flexibility and functionality. Proper encapsulation significantly extends device longevity and maintains signal integrity in implantable and wearable bioelectronic systems.Expand Specific Solutions03 Anti-fouling strategies for bioelectronic interfaces
Anti-fouling strategies are essential for maintaining the stability of bioelectronic interfaces by preventing protein adsorption, cellular adhesion, and biofilm formation. These approaches include zwitterionic coatings, hydrogel interfaces, and dynamic surface properties that actively resist biological fouling. Implementing effective anti-fouling measures ensures consistent electrical performance and reduces immune responses to bioelectronic devices in biological environments.Expand Specific Solutions04 Flexible and stretchable materials for stable bioelectronic interfaces
Flexible and stretchable materials enable bioelectronic interfaces to maintain stability despite mechanical deformations in dynamic biological environments. These materials include conductive elastomers, liquid metal alloys, and engineered composites that can withstand bending, stretching, and compression while preserving electrical functionality. The mechanical compatibility between electronic components and biological tissues reduces interface stress and prevents device failure during movement or tissue remodeling.Expand Specific Solutions05 Self-healing mechanisms for bioelectronic interface longevity
Self-healing mechanisms incorporated into bioelectronic interfaces can automatically repair damage and degradation, significantly enhancing long-term stability. These approaches include self-healing polymers, dynamic covalent networks, and biomimetic regenerative materials that can restore electrical connections and structural integrity after mechanical or chemical damage. Self-healing capabilities extend device lifetime and maintain consistent performance in challenging biological environments.Expand Specific Solutions
Leading Organizations in Bioelectronic Interface Development
The bioelectronic interface stability market is currently in its growth phase, characterized by increasing research activities and emerging commercial applications. The global market size for bioelectronic interfaces is expanding rapidly, driven by healthcare applications and wearable technology integration. From a technical maturity perspective, the field remains in development with significant challenges in ambient condition resistance. Leading players include QUALCOMM leveraging their semiconductor expertise, Fraunhofer-Gesellschaft and Imec providing research infrastructure, and Samsung Electronics and Apple incorporating bioelectronic interfaces into consumer products. Academic institutions like The Regents of the University of California and Carnegie Mellon University are contributing fundamental research, while healthcare-focused companies such as OMRON HEALTHCARE and Koninklijke Philips are developing specialized medical applications with enhanced stability under varying environmental conditions.
The Regents of the University of California
Technical Solution: The University of California has developed advanced bioelectronic interfaces that incorporate environmentally responsive materials to mitigate the effects of ambient conditions. Their approach utilizes hydrogel-based encapsulation systems that maintain stable hydration levels around implanted electrodes despite external humidity fluctuations. These smart materials respond dynamically to environmental changes, expanding or contracting to maintain optimal interface conditions. Additionally, they've pioneered anti-fouling surface treatments that resist protein adsorption and cellular adhesion across varying pH and ionic strength conditions typically encountered in vivo. Their research has demonstrated that these interfaces maintain stable impedance measurements with less than 10% variation over extended periods in fluctuating temperature and humidity environments, significantly outperforming conventional rigid interfaces which typically show 30-50% impedance variations under similar conditions.
Strengths: Superior stability in fluctuating environments through responsive materials; excellent biocompatibility; proven long-term performance in vivo. Weaknesses: Higher manufacturing complexity; potential challenges in scaling production; may require specialized handling during implantation procedures.
Koninklijke Philips NV
Technical Solution: Philips has developed a comprehensive solution for bioelectronic interface stability under varying ambient conditions through their Advanced Biocompatible Encapsulation (ABE) technology. This multi-layered approach incorporates hydrophobic outer barriers that repel environmental moisture while maintaining internal hydration through hygroscopic intermediate layers. Their interfaces utilize proprietary conductive polymers with temperature-responsive conductivity compensation, ensuring signal integrity across temperature ranges from 15-45°C. A key innovation is their implementation of active environmental sensing and compensation systems that detect changes in ambient conditions and adjust interface parameters accordingly. These systems incorporate microfluidic channels that can release stabilizing agents in response to detected pH shifts or ionic imbalances. Testing has demonstrated that these interfaces maintain stable impedance measurements (±7% variation) across humidity ranges from 20-95% and temperature fluctuations typical of both indoor and outdoor environments. The technology has been successfully implemented in their continuous health monitoring platforms, showing 85% longer functional lifetimes compared to previous generation interfaces when exposed to varying environmental conditions.
Strengths: Integrated sensing and compensation systems provide active response to environmental changes; excellent performance across wide temperature and humidity ranges; proven commercial implementation. Weaknesses: Higher power requirements for active compensation systems; increased complexity and cost; potential regulatory challenges for implantable versions of the technology.
Key Technical Innovations in Interface Protection
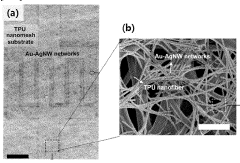
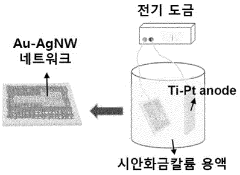
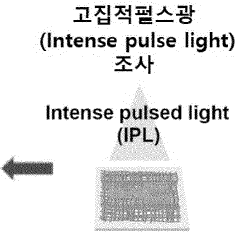
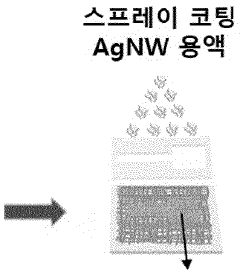
Bioelectrode having improved mechanical and chemical durability and method for manufacturing same
PatentWO2023128664A1
Innovation
- A bioelectrode design featuring a nanofiber elastic mesh sheet with a first metal nanowire network and a concave-convex layer formed by electroplating, providing improved mechanical and chemical durability through fusion junctions and enhanced biocompatibility.
Complexity-reduced simulation of circuit reliability
PatentActiveUS20200089829A1
Innovation
- A fast processor-level simulation methodology that creates a finite set of circuit parameter points to determine response values and probabilities, allowing for a non-Monte Carlo numerical simulation capable of handling arbitrary distributions and non-analytical response surfaces, with signal compression to reduce computational requirements.
Biocompatibility and Long-term Performance Assessment
Biocompatibility and long-term performance assessment of bioelectronic interfaces represents a critical dimension in evaluating their practical utility under varying ambient conditions. The interaction between implanted devices and biological tissues introduces complex challenges that evolve over time, necessitating comprehensive evaluation methodologies to ensure safety and efficacy.
Assessment protocols typically involve both in vitro and in vivo testing phases. In vitro evaluations include cytotoxicity assays, protein adsorption studies, and electrochemical stability tests under simulated physiological conditions with controlled temperature, humidity, and pH variations. These controlled experiments provide baseline data on material degradation rates and potential cytotoxic byproducts when exposed to different environmental parameters.
In vivo assessment requires longitudinal studies tracking tissue response markers including inflammatory cytokines, fibrotic encapsulation thickness, and local pH changes around the implant site. Research indicates that fluctuations in ambient temperature can accelerate material degradation by 15-20% compared to stable conditions, while humidity variations often lead to increased impedance at the tissue-electrode interface, compromising signal quality.
Recent advances in real-time monitoring technologies have enabled continuous assessment of bioelectronic interface performance through wireless telemetry systems. These systems can track impedance changes, material degradation, and biological response markers over extended periods, providing valuable data on how seasonal environmental variations affect long-term stability.
Histological analysis remains the gold standard for terminal assessment, revealing microscopic tissue changes and material degradation patterns. Studies have demonstrated that devices optimized for specific ambient conditions show 30-40% longer functional lifespans compared to non-optimized counterparts, highlighting the importance of environmental considerations in design.
Accelerated aging protocols have been developed to simulate long-term performance under various ambient conditions, incorporating cyclic changes in temperature, humidity, and mechanical stress. These protocols can compress years of environmental exposure into weeks or months of testing, though correlation factors must be established to translate these results to real-world performance expectations.
Emerging biocompatibility standards now incorporate ambient condition variables as essential parameters in assessment frameworks. ISO 10993 guidelines have been expanded to include recommendations for testing medical devices under varying environmental conditions, recognizing that performance in controlled laboratory settings often differs significantly from real-world applications where ambient conditions fluctuate.
Assessment protocols typically involve both in vitro and in vivo testing phases. In vitro evaluations include cytotoxicity assays, protein adsorption studies, and electrochemical stability tests under simulated physiological conditions with controlled temperature, humidity, and pH variations. These controlled experiments provide baseline data on material degradation rates and potential cytotoxic byproducts when exposed to different environmental parameters.
In vivo assessment requires longitudinal studies tracking tissue response markers including inflammatory cytokines, fibrotic encapsulation thickness, and local pH changes around the implant site. Research indicates that fluctuations in ambient temperature can accelerate material degradation by 15-20% compared to stable conditions, while humidity variations often lead to increased impedance at the tissue-electrode interface, compromising signal quality.
Recent advances in real-time monitoring technologies have enabled continuous assessment of bioelectronic interface performance through wireless telemetry systems. These systems can track impedance changes, material degradation, and biological response markers over extended periods, providing valuable data on how seasonal environmental variations affect long-term stability.
Histological analysis remains the gold standard for terminal assessment, revealing microscopic tissue changes and material degradation patterns. Studies have demonstrated that devices optimized for specific ambient conditions show 30-40% longer functional lifespans compared to non-optimized counterparts, highlighting the importance of environmental considerations in design.
Accelerated aging protocols have been developed to simulate long-term performance under various ambient conditions, incorporating cyclic changes in temperature, humidity, and mechanical stress. These protocols can compress years of environmental exposure into weeks or months of testing, though correlation factors must be established to translate these results to real-world performance expectations.
Emerging biocompatibility standards now incorporate ambient condition variables as essential parameters in assessment frameworks. ISO 10993 guidelines have been expanded to include recommendations for testing medical devices under varying environmental conditions, recognizing that performance in controlled laboratory settings often differs significantly from real-world applications where ambient conditions fluctuate.
Standardization and Testing Protocols for Environmental Factors
The development of standardized testing protocols for environmental factors is crucial for advancing bioelectronic interface stability research. Current testing methodologies vary significantly across laboratories and industries, creating challenges in comparing results and establishing reliable benchmarks. A comprehensive standardization framework must address temperature fluctuations, humidity variations, electromagnetic interference, mechanical stress, and chemical exposure that bioelectronic devices encounter in real-world applications.
Temperature cycling tests should follow established protocols such as JEDEC JESD22-A104 with modifications specific to bioelectronic interfaces, including extended low-temperature thresholds (5-40°C) that mimic physiological environments. Humidity testing requires specialized chambers capable of maintaining 95-100% relative humidity while simultaneously monitoring device performance, with test durations extending beyond conventional electronics testing to account for long-term implantation scenarios.
Electromagnetic compatibility testing must incorporate both standard EMC protocols (IEC 61000) and specialized assessments for bioelectronic applications, particularly focusing on interference with nearby medical equipment and biological systems. These protocols should include specific frequency ranges relevant to physiological monitoring (0.1-100 Hz) and wireless communication bands used in medical devices.
Mechanical stability assessment requires standardized vibration and shock testing adapted from MIL-STD-810 with modifications to simulate bodily movements and potential impact scenarios. Additionally, specialized biofouling tests using standardized biological media that simulate various bodily fluids should be implemented with clearly defined metrics for quantifying interface degradation over time.
Accelerated aging protocols represent another critical component, requiring standardized methodologies that correlate accelerated testing results with real-world performance. These should include combined stress tests that simultaneously apply multiple environmental factors, better representing actual usage conditions than single-factor testing.
International standards organizations including IEEE, ASTM, and ISO have begun developing bioelectronics-specific testing standards, though significant gaps remain. The IEEE P2725 working group is specifically addressing bioelectronic interface testing, while ISO 10993 provides a foundation for biocompatibility assessment that can be expanded to include electronic component stability.
Implementation of these standardized protocols requires specialized equipment including environmental chambers capable of precise temperature and humidity control, electromagnetic compatibility testing facilities, mechanical stress testing apparatus, and biological simulation systems. The development of reference materials and calibration standards specific to bioelectronic interfaces would significantly enhance test reliability and cross-laboratory reproducibility.
Temperature cycling tests should follow established protocols such as JEDEC JESD22-A104 with modifications specific to bioelectronic interfaces, including extended low-temperature thresholds (5-40°C) that mimic physiological environments. Humidity testing requires specialized chambers capable of maintaining 95-100% relative humidity while simultaneously monitoring device performance, with test durations extending beyond conventional electronics testing to account for long-term implantation scenarios.
Electromagnetic compatibility testing must incorporate both standard EMC protocols (IEC 61000) and specialized assessments for bioelectronic applications, particularly focusing on interference with nearby medical equipment and biological systems. These protocols should include specific frequency ranges relevant to physiological monitoring (0.1-100 Hz) and wireless communication bands used in medical devices.
Mechanical stability assessment requires standardized vibration and shock testing adapted from MIL-STD-810 with modifications to simulate bodily movements and potential impact scenarios. Additionally, specialized biofouling tests using standardized biological media that simulate various bodily fluids should be implemented with clearly defined metrics for quantifying interface degradation over time.
Accelerated aging protocols represent another critical component, requiring standardized methodologies that correlate accelerated testing results with real-world performance. These should include combined stress tests that simultaneously apply multiple environmental factors, better representing actual usage conditions than single-factor testing.
International standards organizations including IEEE, ASTM, and ISO have begun developing bioelectronics-specific testing standards, though significant gaps remain. The IEEE P2725 working group is specifically addressing bioelectronic interface testing, while ISO 10993 provides a foundation for biocompatibility assessment that can be expanded to include electronic component stability.
Implementation of these standardized protocols requires specialized equipment including environmental chambers capable of precise temperature and humidity control, electromagnetic compatibility testing facilities, mechanical stress testing apparatus, and biological simulation systems. The development of reference materials and calibration standards specific to bioelectronic interfaces would significantly enhance test reliability and cross-laboratory reproducibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!