Trends in Bioelectronic Interface Regulation Across the EU
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Regulatory Evolution and Objectives
Bioelectronic interfaces represent a rapidly evolving field at the intersection of biology, electronics, and medicine. The European Union has been at the forefront of developing comprehensive regulatory frameworks for these technologies, recognizing their transformative potential in healthcare and beyond. The evolution of bioelectronic interface regulation in the EU can be traced back to the early 2000s, when initial guidelines focused primarily on basic medical device safety standards without specific provisions for neural interfaces or bioelectronic systems.
By 2010, the increasing sophistication of implantable neural devices and brain-computer interfaces prompted the European Medicines Agency (EMA) to establish specialized working groups dedicated to addressing the unique challenges posed by these technologies. This marked a significant shift from general medical device regulation to more specialized governance frameworks that acknowledged the distinctive nature of direct biological-electronic interactions.
The 2017 Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) represented watershed moments in EU bioelectronic regulation, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification specifically addressing implantable electronic devices. These regulations established a foundation for the governance of increasingly sophisticated bioelectronic interfaces while emphasizing patient safety and data protection.
Recent regulatory developments have focused on addressing the ethical implications of bioelectronic interfaces, particularly concerning neural data privacy, cognitive liberty, and informed consent. The EU's approach has evolved toward a risk-based regulatory framework that balances innovation enablement with appropriate safeguards for human dignity and autonomy. This reflects the growing recognition that bioelectronic interfaces raise unique ethical and philosophical questions beyond traditional medical device concerns.
The current regulatory objectives in the EU center on several key priorities: establishing harmonized standards for bioelectronic interface safety and performance; developing specialized protocols for clinical validation of neural interfaces; creating frameworks for the responsible collection, storage, and use of neural data; and ensuring equitable access to bioelectronic healthcare innovations across member states.
Looking forward, the EU aims to position itself as a global leader in responsible bioelectronic innovation through its regulatory approach. This includes developing anticipatory governance mechanisms that can adapt to rapidly evolving technologies while maintaining core ethical principles. The European Commission's Horizon Europe program has identified bioelectronic medicine as a strategic priority, with regulatory science initiatives designed to keep pace with technological advancement in this field.
By 2010, the increasing sophistication of implantable neural devices and brain-computer interfaces prompted the European Medicines Agency (EMA) to establish specialized working groups dedicated to addressing the unique challenges posed by these technologies. This marked a significant shift from general medical device regulation to more specialized governance frameworks that acknowledged the distinctive nature of direct biological-electronic interactions.
The 2017 Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) represented watershed moments in EU bioelectronic regulation, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification specifically addressing implantable electronic devices. These regulations established a foundation for the governance of increasingly sophisticated bioelectronic interfaces while emphasizing patient safety and data protection.
Recent regulatory developments have focused on addressing the ethical implications of bioelectronic interfaces, particularly concerning neural data privacy, cognitive liberty, and informed consent. The EU's approach has evolved toward a risk-based regulatory framework that balances innovation enablement with appropriate safeguards for human dignity and autonomy. This reflects the growing recognition that bioelectronic interfaces raise unique ethical and philosophical questions beyond traditional medical device concerns.
The current regulatory objectives in the EU center on several key priorities: establishing harmonized standards for bioelectronic interface safety and performance; developing specialized protocols for clinical validation of neural interfaces; creating frameworks for the responsible collection, storage, and use of neural data; and ensuring equitable access to bioelectronic healthcare innovations across member states.
Looking forward, the EU aims to position itself as a global leader in responsible bioelectronic innovation through its regulatory approach. This includes developing anticipatory governance mechanisms that can adapt to rapidly evolving technologies while maintaining core ethical principles. The European Commission's Horizon Europe program has identified bioelectronic medicine as a strategic priority, with regulatory science initiatives designed to keep pace with technological advancement in this field.
EU Market Demand Analysis for Bioelectronic Technologies
The European Union bioelectronic technologies market demonstrates robust growth potential, driven by increasing healthcare demands and technological advancements. Current market assessments value the EU bioelectronic medical devices sector at approximately 15 billion euros, with projected annual growth rates between 7-9% through 2030, significantly outpacing traditional medical technology segments.
Demographic shifts across Europe substantially influence market dynamics, with an aging population creating heightened demand for innovative healthcare solutions. Currently, 20% of EU citizens are over 65, expected to reach 30% by 2050. This demographic transition creates particular demand for bioelectronic technologies addressing age-related conditions including neurodegenerative disorders, cardiovascular diseases, and mobility impairments.
Healthcare expenditure patterns further validate market potential, with EU member states collectively allocating over 1.5 trillion euros annually to healthcare, representing nearly 10% of the region's GDP. Bioelectronic technologies align with European healthcare systems' increasing focus on cost-effective, preventative, and personalized treatment approaches that reduce hospitalization requirements and improve patient outcomes.
Consumer acceptance of bioelectronic interfaces shows promising trends, with recent surveys indicating 68% of European patients express willingness to use implantable or wearable bioelectronic devices if recommended by healthcare providers. This acceptance rate has increased by 15 percentage points over the past five years, signaling growing public comfort with these technologies.
Regional variations exist within the EU market, with Northern and Western European countries demonstrating higher adoption rates compared to Southern and Eastern regions. Germany, France, the Netherlands, and Scandinavian countries represent the most developed markets, accounting for approximately 65% of current bioelectronic technology implementation.
Institutional demand presents another significant market driver, with over 12,000 hospitals and 80,000 healthcare facilities across the EU seeking advanced technological solutions to improve care delivery efficiency. The European Commission's Digital Health Strategy and Horizon Europe program have allocated substantial funding—exceeding 4 billion euros—specifically for digital health innovations including bioelectronic interfaces.
Regulatory harmonization efforts across the EU create a more predictable market environment, though challenges remain in standardizing approval processes. The Medical Device Regulation (MDR) implementation has established clearer pathways for bioelectronic technology certification, potentially accelerating market entry timelines by 30-40% compared to previous regulatory frameworks.
Demographic shifts across Europe substantially influence market dynamics, with an aging population creating heightened demand for innovative healthcare solutions. Currently, 20% of EU citizens are over 65, expected to reach 30% by 2050. This demographic transition creates particular demand for bioelectronic technologies addressing age-related conditions including neurodegenerative disorders, cardiovascular diseases, and mobility impairments.
Healthcare expenditure patterns further validate market potential, with EU member states collectively allocating over 1.5 trillion euros annually to healthcare, representing nearly 10% of the region's GDP. Bioelectronic technologies align with European healthcare systems' increasing focus on cost-effective, preventative, and personalized treatment approaches that reduce hospitalization requirements and improve patient outcomes.
Consumer acceptance of bioelectronic interfaces shows promising trends, with recent surveys indicating 68% of European patients express willingness to use implantable or wearable bioelectronic devices if recommended by healthcare providers. This acceptance rate has increased by 15 percentage points over the past five years, signaling growing public comfort with these technologies.
Regional variations exist within the EU market, with Northern and Western European countries demonstrating higher adoption rates compared to Southern and Eastern regions. Germany, France, the Netherlands, and Scandinavian countries represent the most developed markets, accounting for approximately 65% of current bioelectronic technology implementation.
Institutional demand presents another significant market driver, with over 12,000 hospitals and 80,000 healthcare facilities across the EU seeking advanced technological solutions to improve care delivery efficiency. The European Commission's Digital Health Strategy and Horizon Europe program have allocated substantial funding—exceeding 4 billion euros—specifically for digital health innovations including bioelectronic interfaces.
Regulatory harmonization efforts across the EU create a more predictable market environment, though challenges remain in standardizing approval processes. The Medical Device Regulation (MDR) implementation has established clearer pathways for bioelectronic technology certification, potentially accelerating market entry timelines by 30-40% compared to previous regulatory frameworks.
Current Regulatory Framework and Challenges in the EU
The European Union has established a complex regulatory framework for bioelectronic interfaces, primarily governed by the Medical Device Regulation (MDR 2017/745) and the In Vitro Diagnostic Regulation (IVDR 2017/746). These regulations, which replaced the previous Medical Device Directive, represent a significant evolution in the EU's approach to regulating emerging bioelectronic technologies. The MDR specifically addresses implantable devices and active medical devices that interface with the human nervous system, imposing stringent requirements for clinical evaluation, risk management, and post-market surveillance.
A key feature of the current framework is the classification system that categorizes bioelectronic interfaces based on their risk profile. Class III devices, which include most invasive neural interfaces and brain-computer interfaces, face the most rigorous conformity assessment procedures before receiving CE marking. This classification system has created a graduated regulatory approach that attempts to balance innovation with patient safety.
The General Data Protection Regulation (GDPR) intersects significantly with bioelectronic interface regulation, particularly regarding the processing of neural data and other biometric information. This creates a dual regulatory burden where manufacturers must comply with both medical device regulations and data protection requirements, especially for devices that collect, process, or transmit neural signals.
Despite the comprehensive nature of the EU regulatory framework, several challenges persist. The rapid pace of technological advancement in bioelectronics often outstrips regulatory adaptation, creating uncertainty for innovators. Many bioelectronic interfaces exist in regulatory gray zones, particularly those with both therapeutic and enhancement applications, which the current framework struggles to categorize appropriately.
Harmonization issues across member states present another significant challenge. While the MDR aims to standardize requirements across the EU, national interpretations and implementation timelines vary considerably. This creates a fragmented regulatory landscape that complicates market access strategies for bioelectronic device manufacturers.
The regulatory pathway for novel bioelectronic interfaces remains unclear in many cases, with notified bodies often lacking specialized expertise to evaluate cutting-edge neural technologies. This has created bottlenecks in the conformity assessment process, delaying innovation and market access.
Ethical considerations represent perhaps the most complex challenge in the current framework. Issues surrounding autonomy, identity, and cognitive liberty are inadequately addressed in existing regulations, which focus primarily on physical safety and performance rather than the unique ethical implications of technologies that interface directly with the human brain and nervous system.
A key feature of the current framework is the classification system that categorizes bioelectronic interfaces based on their risk profile. Class III devices, which include most invasive neural interfaces and brain-computer interfaces, face the most rigorous conformity assessment procedures before receiving CE marking. This classification system has created a graduated regulatory approach that attempts to balance innovation with patient safety.
The General Data Protection Regulation (GDPR) intersects significantly with bioelectronic interface regulation, particularly regarding the processing of neural data and other biometric information. This creates a dual regulatory burden where manufacturers must comply with both medical device regulations and data protection requirements, especially for devices that collect, process, or transmit neural signals.
Despite the comprehensive nature of the EU regulatory framework, several challenges persist. The rapid pace of technological advancement in bioelectronics often outstrips regulatory adaptation, creating uncertainty for innovators. Many bioelectronic interfaces exist in regulatory gray zones, particularly those with both therapeutic and enhancement applications, which the current framework struggles to categorize appropriately.
Harmonization issues across member states present another significant challenge. While the MDR aims to standardize requirements across the EU, national interpretations and implementation timelines vary considerably. This creates a fragmented regulatory landscape that complicates market access strategies for bioelectronic device manufacturers.
The regulatory pathway for novel bioelectronic interfaces remains unclear in many cases, with notified bodies often lacking specialized expertise to evaluate cutting-edge neural technologies. This has created bottlenecks in the conformity assessment process, delaying innovation and market access.
Ethical considerations represent perhaps the most complex challenge in the current framework. Issues surrounding autonomy, identity, and cognitive liberty are inadequately addressed in existing regulations, which focus primarily on physical safety and performance rather than the unique ethical implications of technologies that interface directly with the human brain and nervous system.
Current EU Compliance Approaches for Bioelectronic Interfaces
01 Neural interfaces for bioelectronic applications
Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neural tissue, or both. They are crucial for applications such as brain-computer interfaces, neural prosthetics, and neuromodulation therapies. Advanced materials and fabrication techniques are employed to create biocompatible interfaces that minimize tissue damage and immune response while maintaining long-term functionality.- Neural interfaces for bioelectronic applications: Neural interfaces are designed to establish direct communication between electronic devices and the nervous system. These interfaces can record neural activity, stimulate neurons, or both, enabling applications in neuroprosthetics, brain-computer interfaces, and treatment of neurological disorders. Advanced materials and fabrication techniques are used to create biocompatible electrodes that can effectively interface with neural tissue while minimizing tissue damage and immune response.
- Flexible and stretchable bioelectronic interfaces: Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic surfaces of biological tissues. These interfaces incorporate elastic materials, serpentine structures, or mesh designs to accommodate movement while maintaining electrical functionality. Such interfaces reduce mechanical mismatch between rigid electronics and soft tissues, improving long-term stability and reducing inflammation. Applications include wearable health monitors, implantable sensors, and electronic skin for prosthetics.
- Biosensing and molecular detection interfaces: Bioelectronic interfaces for biosensing and molecular detection utilize various transduction mechanisms to convert biological signals into measurable electrical outputs. These interfaces incorporate recognition elements such as antibodies, enzymes, or nucleic acids to selectively bind target analytes. Signal amplification strategies and nanomaterials enhance sensitivity and specificity. Applications include point-of-care diagnostics, continuous health monitoring, and environmental sensing of biological agents.
- Implantable bioelectronic medical devices: Implantable bioelectronic medical devices integrate electronic components with biological systems for therapeutic or diagnostic purposes. These devices include neural stimulators, drug delivery systems, and physiological monitors that can be placed within the body for extended periods. Advanced encapsulation techniques protect electronic components from the harsh biological environment while allowing for signal transmission. Power management systems, including wireless power transfer and energy harvesting, enable long-term operation without battery replacement.
- Organic and biomaterial-based electronic interfaces: Organic and biomaterial-based electronic interfaces utilize conductive polymers, proteins, or other biological materials as active components in electronic devices. These materials offer advantages in biocompatibility, biodegradability, and mechanical properties that more closely match biological tissues. Fabrication techniques include printing, self-assembly, and enzymatic synthesis. Applications include transient electronics that dissolve after use, environmentally friendly electronic components, and interfaces that can integrate with living tissue with minimal foreign body response.
02 Flexible and stretchable bioelectronic interfaces
Flexible and stretchable bioelectronic interfaces are designed to conform to the dynamic nature of biological tissues. These interfaces utilize elastic materials, serpentine structures, or mesh designs to accommodate movement while maintaining electrical functionality. They reduce mechanical mismatch between rigid electronics and soft tissues, minimizing inflammation and improving long-term stability. Applications include epidermal electronics, implantable sensors, and conformable neural interfaces that can adapt to tissue movement and growth.Expand Specific Solutions03 Biosensing interfaces for molecular detection
Biosensing interfaces integrate biological recognition elements with electronic transduction mechanisms to detect specific molecules or biological events. These interfaces employ various sensing modalities including electrochemical, optical, and piezoelectric methods to convert biological interactions into measurable electronic signals. They can be functionalized with antibodies, enzymes, nucleic acids, or other biomolecules to achieve high specificity. Applications include point-of-care diagnostics, continuous health monitoring, and environmental sensing.Expand Specific Solutions04 Implantable bioelectronic interfaces for therapeutic applications
Implantable bioelectronic interfaces are designed for long-term integration with living tissues to deliver therapeutic interventions. These devices can monitor physiological parameters, deliver electrical stimulation, or release drugs in response to specific biological signals. Advanced encapsulation techniques protect electronic components from the harsh biological environment while allowing for signal transduction. Applications include cardiac pacemakers, deep brain stimulators, spinal cord stimulators, and closed-loop drug delivery systems.Expand Specific Solutions05 Nanomaterial-based bioelectronic interfaces
Nanomaterial-based bioelectronic interfaces leverage the unique properties of nanomaterials to enhance the performance of bioelectronic devices. Materials such as carbon nanotubes, graphene, quantum dots, and metal nanoparticles provide improved electrical conductivity, increased surface area, and enhanced biocompatibility. These interfaces enable higher sensitivity in biosensing applications, improved charge injection capacity for neural stimulation, and reduced dimensions for minimally invasive implantation. They can be functionalized with biomolecules to improve specificity and tissue integration.Expand Specific Solutions
Key Regulatory Bodies and Industry Stakeholders
The bioelectronic interface regulation landscape across the EU is evolving rapidly, currently in a transitional growth phase characterized by increasing market adoption but fragmented regulatory frameworks. The market is projected to reach significant scale as healthcare applications expand, though technical standardization remains a challenge. Leading academic institutions (MIT, Johns Hopkins, Zhejiang University) are driving fundamental research, while established technology corporations (Samsung, Qualcomm, Huawei) are commercializing applications. Specialized biotech firms like SenzaGen and microLIQUID are developing niche solutions. The regulatory maturity varies across EU member states, with efforts underway to harmonize standards for safety, data privacy, and interoperability as the technology advances from experimental to clinical implementation.
Conectate Soluciones y Aplicaciones SL
Technical Solution: Conectate Soluciones y Aplicaciones has developed a comprehensive regulatory navigation platform specifically for bioelectronic interfaces in the EU market. Their approach integrates regulatory requirements from multiple EU directives and regulations that impact bioelectronic technologies, including the Medical Device Regulation (MDR), In Vitro Diagnostic Regulation (IVDR), and General Data Protection Regulation (GDPR). The company has created a specialized digital platform that guides bioelectronic device manufacturers through the complex regulatory landscape, providing customized compliance pathways based on device classification, intended use, and risk profile. Their system incorporates real-time regulatory updates from across EU member states, ensuring that manufacturers remain compliant with evolving requirements. Conectate has also developed specialized modules for bioelectronic interfaces that address unique challenges such as neural data protection, long-term biocompatibility, and electromagnetic compatibility requirements specific to devices that interface with biological systems. Their approach emphasizes early regulatory consideration during the design phase to streamline approval processes and reduce time-to-market for innovative bioelectronic technologies.
Strengths: Comprehensive digital platform specifically designed for bioelectronic regulatory navigation; real-time regulatory intelligence across EU member states; practical implementation focus for manufacturers. Weaknesses: Limited research contribution to regulatory science compared to academic institutions; dependence on timely updates from regulatory authorities across multiple jurisdictions.
Universita Degli Studi di Cagliari
Technical Solution: The University of Cagliari has developed a comprehensive approach to bioelectronic interface regulation in the EU, focusing on neural interfaces and implantable medical devices. Their research team has pioneered a regulatory framework that addresses both technical and ethical considerations specific to the European context. They have established the BioElectronics Regulatory Innovation Lab that collaborates directly with the European Medicines Agency (EMA) and national regulatory bodies to develop standardized testing protocols for bioelectronic devices. Their approach emphasizes patient safety while balancing innovation needs, with particular attention to GDPR compliance for neural data collection and processing. The university has also contributed to the development of the EU Medical Device Regulation (MDR) guidelines specific to bioelectronic interfaces, providing technical specifications for biocompatibility, electrical safety, and long-term stability requirements.
Strengths: Strong integration with EU regulatory bodies and direct influence on policy development; specialized expertise in GDPR compliance for neural interfaces. Weaknesses: Limited commercial application compared to industry players; research focus may not always align with market demands and implementation challenges.
Critical EU Directives and Standards for Bioelectronics
Organic transistor-based system for electrophysiological monitoring of cells and method for the monitoring of the cells
PatentActiveUS20180031520A1
Innovation
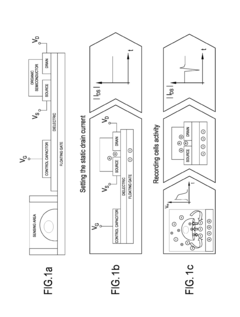
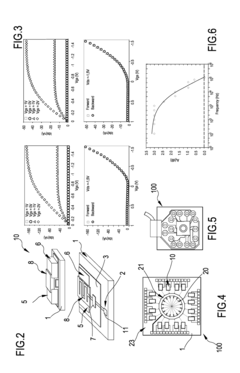
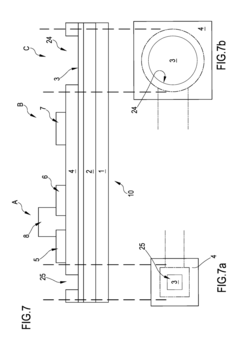
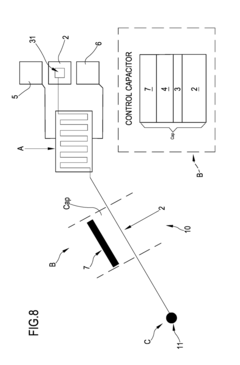
- A system comprising a plurality of organic thin film transistors with a floating gate electrode, source and drain electrodes, and an insulating layer, operated at low voltages (0.5 V to 2 V) to detect dynamic charge variations in the frequency range of cell electrical activity (1 Hz to 1000 Hz) without an external reference electrode, using a biocompatible sensing area with apertures to expose floating gates to cells, allowing for spatial mapping of cell activity.
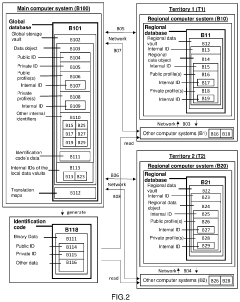
Procedure for unified global registry and universal identification of products of biological origin for medicinal purposes
PatentActiveUS20210183479A1
Innovation
- A unified global registry and universal identification system using a software application to create a global and unique data storage vault, with regional data vaults and profiles, employing unique identifiers for products and individuals, enabling cross-border traceability and quality control.
Cross-Border Harmonization of Bioelectronic Regulations
The harmonization of bioelectronic interface regulations across EU member states represents a critical challenge in the evolving landscape of medical technology governance. Currently, significant regulatory disparities exist between countries, creating barriers to market entry and slowing innovation in the bioelectronics sector. These differences manifest in varying approval processes, safety standards, and post-market surveillance requirements, which collectively impede the seamless deployment of bioelectronic solutions across the European market.
Recent initiatives by the European Commission have begun addressing these challenges through the development of unified regulatory frameworks. The Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) serve as foundational elements for this harmonization effort, though their implementation specifically for bioelectronic interfaces remains inconsistent across member states.
Cross-border data sharing presents another significant harmonization challenge. Bioelectronic interfaces often generate sensitive health data that must be transmitted across national boundaries, requiring alignment with both the General Data Protection Regulation (GDPR) and country-specific health data regulations. The European Medicines Agency has established working groups focused on creating standardized protocols for bioelectronic data management, though adoption remains fragmented.
Technical standardization efforts have accelerated through collaborations between the European Committee for Standardization (CEN) and the European Committee for Electrotechnical Standardization (CENELEC). These organizations are developing unified technical specifications for bioelectronic interfaces, addressing aspects such as biocompatibility, electrical safety, and electromagnetic compatibility. The ISO/IEEE 11073 standards family has been increasingly adopted as a common framework, though implementation varies by region.
Regulatory reciprocity agreements between member states represent a promising approach to harmonization. The Nordic Mutual Recognition Agreement for medical technologies serves as a successful model, allowing approved bioelectronic devices in one participating country to receive expedited review in others. Similar bilateral and multilateral agreements are emerging throughout the EU, creating a patchwork of regulatory cooperation that may eventually coalesce into a unified system.
The European Commission's Digital Health Network has established a dedicated Bioelectronics Working Group tasked with developing a roadmap for complete regulatory harmonization by 2027. This initiative aims to create a single approval pathway for bioelectronic interfaces while maintaining appropriate safety standards and accommodating the rapid pace of technological innovation in this field.
Recent initiatives by the European Commission have begun addressing these challenges through the development of unified regulatory frameworks. The Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) serve as foundational elements for this harmonization effort, though their implementation specifically for bioelectronic interfaces remains inconsistent across member states.
Cross-border data sharing presents another significant harmonization challenge. Bioelectronic interfaces often generate sensitive health data that must be transmitted across national boundaries, requiring alignment with both the General Data Protection Regulation (GDPR) and country-specific health data regulations. The European Medicines Agency has established working groups focused on creating standardized protocols for bioelectronic data management, though adoption remains fragmented.
Technical standardization efforts have accelerated through collaborations between the European Committee for Standardization (CEN) and the European Committee for Electrotechnical Standardization (CENELEC). These organizations are developing unified technical specifications for bioelectronic interfaces, addressing aspects such as biocompatibility, electrical safety, and electromagnetic compatibility. The ISO/IEEE 11073 standards family has been increasingly adopted as a common framework, though implementation varies by region.
Regulatory reciprocity agreements between member states represent a promising approach to harmonization. The Nordic Mutual Recognition Agreement for medical technologies serves as a successful model, allowing approved bioelectronic devices in one participating country to receive expedited review in others. Similar bilateral and multilateral agreements are emerging throughout the EU, creating a patchwork of regulatory cooperation that may eventually coalesce into a unified system.
The European Commission's Digital Health Network has established a dedicated Bioelectronics Working Group tasked with developing a roadmap for complete regulatory harmonization by 2027. This initiative aims to create a single approval pathway for bioelectronic interfaces while maintaining appropriate safety standards and accommodating the rapid pace of technological innovation in this field.
Ethical and Privacy Implications of Bioelectronic Interfaces
The rapid advancement of bioelectronic interfaces across the European Union raises significant ethical and privacy concerns that demand careful consideration. These technologies, which create direct connections between electronic devices and biological systems, particularly the human nervous system, challenge existing ethical frameworks and privacy protections in unprecedented ways.
Privacy concerns are paramount as bioelectronic interfaces can potentially access, record, and transmit highly sensitive neurological data. This data represents perhaps the most intimate form of personal information—direct insights into cognitive processes, emotional states, and even thoughts. The EU's General Data Protection Regulation (GDPR) provides some protections, but questions remain about whether neural data requires specialized regulatory frameworks beyond current provisions.
Informed consent presents another critical ethical challenge. As these technologies become more sophisticated and potentially invasive, ensuring that users fully understand the implications of allowing devices to interface with their nervous systems becomes increasingly complex. Traditional consent models may prove inadequate when dealing with technologies that could potentially influence cognitive processes or alter perception.
The potential for surveillance and unauthorized access to neural data raises serious concerns about autonomy and cognitive liberty. Without robust safeguards, bioelectronic interfaces could enable unprecedented levels of monitoring and potential manipulation of individuals' thought processes, threatening fundamental aspects of human dignity and self-determination that are core values within EU ethical frameworks.
Questions of identity and authenticity also emerge as these technologies advance. When devices can directly influence neural activity, the boundaries between technology-mediated experiences and authentic human experiences become blurred. This raises philosophical questions about personhood and agency that regulatory frameworks must address.
Accessibility and equity considerations cannot be overlooked. If bioelectronic interfaces offer significant therapeutic or enhancement benefits, ensuring equitable access becomes an ethical imperative. The EU faces the challenge of preventing these technologies from exacerbating existing social divides or creating new forms of inequality based on neural capabilities or access to enhancement.
Dual-use concerns also merit attention, as technologies developed for legitimate medical or research purposes could potentially be repurposed for surveillance, coercion, or military applications. The EU's regulatory approach must balance innovation with appropriate safeguards against misuse, particularly in cross-border contexts where regulatory harmonization remains incomplete.
Privacy concerns are paramount as bioelectronic interfaces can potentially access, record, and transmit highly sensitive neurological data. This data represents perhaps the most intimate form of personal information—direct insights into cognitive processes, emotional states, and even thoughts. The EU's General Data Protection Regulation (GDPR) provides some protections, but questions remain about whether neural data requires specialized regulatory frameworks beyond current provisions.
Informed consent presents another critical ethical challenge. As these technologies become more sophisticated and potentially invasive, ensuring that users fully understand the implications of allowing devices to interface with their nervous systems becomes increasingly complex. Traditional consent models may prove inadequate when dealing with technologies that could potentially influence cognitive processes or alter perception.
The potential for surveillance and unauthorized access to neural data raises serious concerns about autonomy and cognitive liberty. Without robust safeguards, bioelectronic interfaces could enable unprecedented levels of monitoring and potential manipulation of individuals' thought processes, threatening fundamental aspects of human dignity and self-determination that are core values within EU ethical frameworks.
Questions of identity and authenticity also emerge as these technologies advance. When devices can directly influence neural activity, the boundaries between technology-mediated experiences and authentic human experiences become blurred. This raises philosophical questions about personhood and agency that regulatory frameworks must address.
Accessibility and equity considerations cannot be overlooked. If bioelectronic interfaces offer significant therapeutic or enhancement benefits, ensuring equitable access becomes an ethical imperative. The EU faces the challenge of preventing these technologies from exacerbating existing social divides or creating new forms of inequality based on neural capabilities or access to enhancement.
Dual-use concerns also merit attention, as technologies developed for legitimate medical or research purposes could potentially be repurposed for surveillance, coercion, or military applications. The EU's regulatory approach must balance innovation with appropriate safeguards against misuse, particularly in cross-border contexts where regulatory harmonization remains incomplete.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!