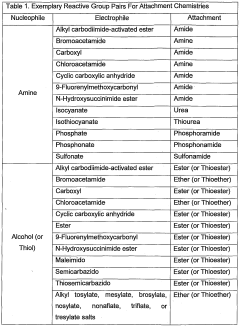
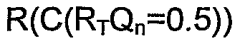Understanding the Electrode Kinetics in Bioelectronic Interfaces
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Evolution and Objectives
Bioelectronic interfaces have evolved significantly over the past several decades, transitioning from rudimentary metal electrodes to sophisticated, multifunctional platforms capable of bidirectional communication with biological systems. The field emerged in the 1950s with the development of basic stimulation electrodes, primarily focused on cardiac pacemakers and early neural stimulation devices. These initial interfaces were characterized by simple designs, limited functionality, and significant biocompatibility challenges.
The 1970s and 1980s witnessed crucial advancements in materials science and microfabrication techniques, enabling the development of more refined electrode arrays with improved spatial resolution. Silicon-based microelectrode arrays (MEAs) emerged during this period, allowing for more precise recording and stimulation capabilities. Concurrently, the understanding of electrode-tissue interactions began to deepen, highlighting the critical role of electrode kinetics in determining interface performance.
By the early 2000s, the field experienced a paradigm shift with the introduction of flexible and stretchable electronics, addressing the mechanical mismatch between rigid electronic components and soft biological tissues. This era also saw the integration of organic electronic materials, which offered improved biocompatibility and novel functionalities such as ion-to-electron transduction capabilities that more closely mimicked biological signaling processes.
Recent developments have focused on enhancing the long-term stability and functionality of bioelectronic interfaces through advanced materials and designs. Conducting polymers, carbon-based materials (including graphene and carbon nanotubes), and hybrid organic-inorganic composites have emerged as promising electrode materials with superior charge transfer characteristics and reduced impedance. These materials facilitate more efficient electrode kinetics, which is crucial for both recording fidelity and stimulation efficacy.
The primary objective in understanding electrode kinetics in bioelectronic interfaces is to optimize the bidirectional signal transduction between electronic devices and biological systems. This involves elucidating the fundamental mechanisms of charge transfer across the electrode-tissue interface, including faradaic and non-faradaic processes, double-layer capacitance effects, and the influence of protein adsorption and cellular encapsulation on long-term electrode performance.
Additional objectives include developing predictive models of electrode behavior in complex biological environments, designing electrodes with tailored kinetic properties for specific applications, and establishing standardized methods for characterizing electrode kinetics in vitro and in vivo. The ultimate goal is to create bioelectronic interfaces that maintain stable, efficient, and biocompatible signal transduction over extended periods, enabling transformative applications in neural prosthetics, bioelectronic medicine, and advanced biosensing platforms.
The 1970s and 1980s witnessed crucial advancements in materials science and microfabrication techniques, enabling the development of more refined electrode arrays with improved spatial resolution. Silicon-based microelectrode arrays (MEAs) emerged during this period, allowing for more precise recording and stimulation capabilities. Concurrently, the understanding of electrode-tissue interactions began to deepen, highlighting the critical role of electrode kinetics in determining interface performance.
By the early 2000s, the field experienced a paradigm shift with the introduction of flexible and stretchable electronics, addressing the mechanical mismatch between rigid electronic components and soft biological tissues. This era also saw the integration of organic electronic materials, which offered improved biocompatibility and novel functionalities such as ion-to-electron transduction capabilities that more closely mimicked biological signaling processes.
Recent developments have focused on enhancing the long-term stability and functionality of bioelectronic interfaces through advanced materials and designs. Conducting polymers, carbon-based materials (including graphene and carbon nanotubes), and hybrid organic-inorganic composites have emerged as promising electrode materials with superior charge transfer characteristics and reduced impedance. These materials facilitate more efficient electrode kinetics, which is crucial for both recording fidelity and stimulation efficacy.
The primary objective in understanding electrode kinetics in bioelectronic interfaces is to optimize the bidirectional signal transduction between electronic devices and biological systems. This involves elucidating the fundamental mechanisms of charge transfer across the electrode-tissue interface, including faradaic and non-faradaic processes, double-layer capacitance effects, and the influence of protein adsorption and cellular encapsulation on long-term electrode performance.
Additional objectives include developing predictive models of electrode behavior in complex biological environments, designing electrodes with tailored kinetic properties for specific applications, and establishing standardized methods for characterizing electrode kinetics in vitro and in vivo. The ultimate goal is to create bioelectronic interfaces that maintain stable, efficient, and biocompatible signal transduction over extended periods, enabling transformative applications in neural prosthetics, bioelectronic medicine, and advanced biosensing platforms.
Market Analysis for Bioelectronic Interface Applications
The bioelectronic interfaces market is experiencing robust growth, driven by increasing applications in neural implants, biosensors, and advanced medical devices. Current market valuations place the global bioelectronics sector at approximately 25 billion USD, with bioelectronic interfaces representing a significant growth segment projected to expand at a compound annual growth rate of 14% through 2030.
Healthcare applications dominate the market landscape, with neural stimulation devices for conditions such as Parkinson's disease, epilepsy, and chronic pain management showing particularly strong demand. The emergence of closed-loop neuromodulation systems that can both record and stimulate neural activity has created a premium segment within this market, valued at over 3 billion USD annually.
Beyond traditional medical applications, consumer-oriented bioelectronic interfaces are gaining traction. Wearable biosensors for continuous health monitoring represent a rapidly expanding market segment, projected to reach 12 billion USD by 2028. These devices increasingly incorporate advanced electrode technologies to improve signal quality and reduce motion artifacts.
The industrial landscape shows regional variations in market focus. North American companies predominantly target therapeutic applications, while Asian manufacturers have established strong positions in biosensing and diagnostic applications. European firms often specialize in high-precision research-grade bioelectronic interfaces for academic and pharmaceutical research applications.
Electrode kinetics represents a critical differentiator in this competitive landscape. Products featuring electrodes with superior charge transfer characteristics command premium pricing, with margins typically 30-40% higher than standard offerings. Market research indicates that customers prioritize signal stability and biocompatibility over initial acquisition costs, creating opportunities for technology-differentiated products.
Regulatory considerations significantly impact market dynamics. Devices with direct neural interfaces face stringent approval processes, creating high barriers to entry but also protecting established players. The FDA's recent guidance on biocompatibility testing for chronically implanted electrodes has accelerated the adoption of novel materials and surface treatments designed to optimize electrode kinetics.
Venture capital investment in bioelectronic interface startups has surged, with over 2 billion USD invested in 2022 alone. Investors are particularly interested in companies developing technologies that address the fundamental challenges of electrode-tissue interfaces, including novel materials that can maintain stable performance over extended implantation periods.
Healthcare applications dominate the market landscape, with neural stimulation devices for conditions such as Parkinson's disease, epilepsy, and chronic pain management showing particularly strong demand. The emergence of closed-loop neuromodulation systems that can both record and stimulate neural activity has created a premium segment within this market, valued at over 3 billion USD annually.
Beyond traditional medical applications, consumer-oriented bioelectronic interfaces are gaining traction. Wearable biosensors for continuous health monitoring represent a rapidly expanding market segment, projected to reach 12 billion USD by 2028. These devices increasingly incorporate advanced electrode technologies to improve signal quality and reduce motion artifacts.
The industrial landscape shows regional variations in market focus. North American companies predominantly target therapeutic applications, while Asian manufacturers have established strong positions in biosensing and diagnostic applications. European firms often specialize in high-precision research-grade bioelectronic interfaces for academic and pharmaceutical research applications.
Electrode kinetics represents a critical differentiator in this competitive landscape. Products featuring electrodes with superior charge transfer characteristics command premium pricing, with margins typically 30-40% higher than standard offerings. Market research indicates that customers prioritize signal stability and biocompatibility over initial acquisition costs, creating opportunities for technology-differentiated products.
Regulatory considerations significantly impact market dynamics. Devices with direct neural interfaces face stringent approval processes, creating high barriers to entry but also protecting established players. The FDA's recent guidance on biocompatibility testing for chronically implanted electrodes has accelerated the adoption of novel materials and surface treatments designed to optimize electrode kinetics.
Venture capital investment in bioelectronic interface startups has surged, with over 2 billion USD invested in 2022 alone. Investors are particularly interested in companies developing technologies that address the fundamental challenges of electrode-tissue interfaces, including novel materials that can maintain stable performance over extended implantation periods.
Current Electrode Kinetics Challenges and Limitations
Despite significant advancements in bioelectronic interfaces, electrode kinetics remains a critical bottleneck in achieving optimal device performance. The fundamental challenge lies in the complex electrochemical reactions occurring at the electrode-tissue interface, where charge transfer mechanisms significantly impact signal quality, device longevity, and biocompatibility. Current electrode materials, predominantly noble metals and carbon-based structures, exhibit limited charge injection capacity, restricting their efficacy in neural stimulation applications.
A major limitation is the formation of the electrical double layer at the electrode-tissue interface, which creates impedance that varies with frequency and degrades over time. This impedance mismatch leads to signal attenuation and distortion, particularly problematic for recording weak biological signals in the microvolt range. Furthermore, the high electrode impedance necessitates increased stimulation voltages, risking tissue damage and electrode degradation through irreversible Faradaic reactions.
Electrode stability presents another significant challenge, as prolonged exposure to the physiological environment triggers material degradation, protein adsorption, and inflammatory responses. These processes alter electrode kinetics over time, compromising long-term recording fidelity and stimulation efficacy. The formation of glial scars around implanted electrodes further increases impedance and distances target neurons, exacerbating signal deterioration.
Current electrode designs face a fundamental trade-off between spatial resolution and signal-to-noise ratio. Miniaturization of electrodes to improve spatial specificity inherently increases impedance, thereby reducing signal quality. This inverse relationship limits the development of high-density electrode arrays necessary for precise neural interface applications.
The heterogeneity of biological tissues introduces additional complexity, as electrode kinetics vary significantly across different tissue types and physiological states. This variability complicates the development of standardized models and predictive frameworks for electrode behavior in vivo, hindering rational design approaches.
Manufacturing challenges further constrain progress, as advanced electrode materials with superior kinetic properties often require complex fabrication processes that are difficult to scale. The integration of novel materials like conducting polymers and nanomaterials, while promising for enhancing charge transfer efficiency, introduces new stability and biocompatibility concerns that remain inadequately addressed.
Analytical limitations also impede advancement, as real-time characterization of electrode kinetics in vivo remains technically challenging. Current methodologies provide limited insight into the dynamic processes occurring at the bioelectronic interface during actual device operation, creating a gap between theoretical models and practical performance.
A major limitation is the formation of the electrical double layer at the electrode-tissue interface, which creates impedance that varies with frequency and degrades over time. This impedance mismatch leads to signal attenuation and distortion, particularly problematic for recording weak biological signals in the microvolt range. Furthermore, the high electrode impedance necessitates increased stimulation voltages, risking tissue damage and electrode degradation through irreversible Faradaic reactions.
Electrode stability presents another significant challenge, as prolonged exposure to the physiological environment triggers material degradation, protein adsorption, and inflammatory responses. These processes alter electrode kinetics over time, compromising long-term recording fidelity and stimulation efficacy. The formation of glial scars around implanted electrodes further increases impedance and distances target neurons, exacerbating signal deterioration.
Current electrode designs face a fundamental trade-off between spatial resolution and signal-to-noise ratio. Miniaturization of electrodes to improve spatial specificity inherently increases impedance, thereby reducing signal quality. This inverse relationship limits the development of high-density electrode arrays necessary for precise neural interface applications.
The heterogeneity of biological tissues introduces additional complexity, as electrode kinetics vary significantly across different tissue types and physiological states. This variability complicates the development of standardized models and predictive frameworks for electrode behavior in vivo, hindering rational design approaches.
Manufacturing challenges further constrain progress, as advanced electrode materials with superior kinetic properties often require complex fabrication processes that are difficult to scale. The integration of novel materials like conducting polymers and nanomaterials, while promising for enhancing charge transfer efficiency, introduces new stability and biocompatibility concerns that remain inadequately addressed.
Analytical limitations also impede advancement, as real-time characterization of electrode kinetics in vivo remains technically challenging. Current methodologies provide limited insight into the dynamic processes occurring at the bioelectronic interface during actual device operation, creating a gap between theoretical models and practical performance.
Contemporary Electrode Kinetics Measurement Techniques
01 Electrode kinetics in electrochemical systems
Electrode kinetics plays a crucial role in electrochemical systems, focusing on the rate of electron transfer at electrode surfaces. These studies examine factors affecting reaction rates such as electrode material, surface properties, and electrolyte composition. Understanding these kinetics is essential for optimizing electrochemical processes, improving efficiency, and enhancing performance in applications like batteries, fuel cells, and sensors.- Electrode kinetics in electrochemical systems: Electrode kinetics plays a crucial role in electrochemical systems, focusing on the rate at which electron transfer occurs at electrode surfaces. These studies examine factors affecting reaction rates such as electrode material, surface conditions, electrolyte composition, and applied potential. Understanding these kinetics is essential for optimizing electrochemical processes, improving efficiency, and developing new technologies for energy storage and conversion applications.
- Measurement and analysis techniques for electrode kinetics: Various techniques are employed to measure and analyze electrode kinetics, including electrochemical impedance spectroscopy, cyclic voltammetry, and chronoamperometry. These methods provide insights into reaction mechanisms, rate constants, and mass transport phenomena. Advanced computational models and algorithms are used to interpret experimental data, enabling researchers to extract kinetic parameters and understand complex electrochemical processes at the electrode-electrolyte interface.
- Electrode materials and surface modifications for enhanced kinetics: The development of novel electrode materials and surface modifications aims to enhance electrode kinetics. These innovations include nanostructured materials, catalytic coatings, and functionalized surfaces that increase active surface area and facilitate faster electron transfer. By optimizing electrode architecture and composition, researchers can achieve improved reaction rates, higher current densities, and better overall performance in applications ranging from batteries to fuel cells and sensors.
- Thermal effects and temperature control in electrode kinetics: Temperature significantly influences electrode kinetics by affecting reaction rates, mass transport, and activation energies. Precise temperature control systems are developed to maintain optimal conditions for electrochemical processes. These systems may include heating elements, cooling mechanisms, and thermal sensors integrated with feedback control algorithms. Understanding and managing thermal effects is crucial for consistent performance, especially in applications where temperature fluctuations can dramatically alter reaction kinetics.
- Electrode kinetics in energy storage and conversion devices: Electrode kinetics is fundamental to the performance of energy storage and conversion devices such as batteries, fuel cells, and supercapacitors. Research focuses on understanding and optimizing charge transfer processes, reducing activation barriers, and enhancing reaction rates at electrode interfaces. Innovations in this area lead to faster charging capabilities, improved power density, extended cycle life, and overall better efficiency in energy devices, addressing key challenges in renewable energy integration and electrification.
02 Measurement techniques for electrode kinetics
Various measurement techniques are employed to study electrode kinetics, including electrochemical impedance spectroscopy, cyclic voltammetry, and chronoamperometry. These methods allow researchers to determine kinetic parameters such as exchange current density, transfer coefficients, and reaction mechanisms. Advanced instrumentation and analytical approaches enable precise characterization of electrode processes under different conditions, providing valuable insights for electrode design and optimization.Expand Specific Solutions03 Electrode kinetics in catalytic reactions
Electrode kinetics significantly impacts catalytic reactions at electrode surfaces. The study focuses on how catalysts affect reaction pathways, activation energies, and electron transfer rates. Understanding these kinetic processes helps in designing more efficient catalysts for applications such as water splitting, CO2 reduction, and fuel cells. The relationship between catalyst structure, composition, and kinetic performance is crucial for developing advanced electrochemical technologies.Expand Specific Solutions04 Modeling and simulation of electrode kinetics
Computational approaches are increasingly used to model and simulate electrode kinetics. These include quantum mechanical calculations, molecular dynamics simulations, and continuum models that predict electron transfer rates and reaction mechanisms. Such theoretical frameworks help researchers understand complex electrochemical processes at the molecular level, guide experimental design, and accelerate the development of improved electrode materials and systems.Expand Specific Solutions05 Temperature effects on electrode kinetics
Temperature significantly influences electrode kinetics by affecting reaction rates, mass transport, and activation energies. Studies in this area examine how thermal conditions impact electron transfer processes, diffusion rates, and overall electrochemical performance. Understanding these temperature dependencies is crucial for designing electrochemical systems that operate efficiently across various thermal environments, particularly important for applications in extreme conditions or where temperature control is critical.Expand Specific Solutions
Leading Research Groups and Companies in Bioelectronics
The bioelectronic interface market is currently in a growth phase, with increasing research focus on electrode kinetics to improve device performance and biocompatibility. The global market is estimated to reach several billion dollars by 2025, driven by applications in neural interfaces, biosensors, and implantable devices. Academic institutions lead fundamental research, with MIT, University of Michigan, and Max Planck Society making significant contributions to understanding electrode-tissue interactions. Commercial players like Infineon Technologies, Philips, and Abbott Cardiovascular Systems are advancing practical applications, focusing on translating research into marketable products. The technology remains in mid-maturity, with ongoing challenges in long-term stability and biocompatibility that require collaborative efforts between academic and industrial partners to overcome existing limitations.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed sophisticated bioelectronic interfaces with a focus on understanding electrode kinetics through their interdisciplinary research approach. Their technology platform incorporates advanced materials science and neural engineering principles to create high-performance electrode systems with enhanced charge transfer capabilities. Michigan researchers have pioneered the development of ultra-small, high-density microelectrode arrays using silicon microfabrication techniques that minimize tissue damage while maximizing recording fidelity. Their electrode designs incorporate specialized surface treatments including electrodeposition of conducting polymers and carbon nanomaterials to significantly reduce impedance and increase charge injection capacity. Michigan's research teams have developed comprehensive computational models of the electrode-tissue interface that account for both electrical and biological factors, enabling prediction of long-term electrode performance. Their recent innovations include shape-memory polymer substrates that allow electrodes to be inserted in a compact form before deploying into their final configuration within neural tissue.
Strengths: Strong interdisciplinary collaboration between engineering, materials science, and neuroscience departments; extensive experience with in vivo testing and validation of electrode technologies. Weaknesses: Some of their most advanced technologies require complex fabrication processes that may present challenges for large-scale manufacturing; academic research focus may sometimes prioritize novel approaches over commercial practicality.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced bioelectronic interfaces focusing on electrode kinetics through their specialized materials engineering approach. Their research teams have pioneered conductive polymer-based electrodes with enhanced charge transfer capabilities at the tissue-electrode interface. MIT's technology utilizes PEDOT:PSS (poly(3,4-ethylenedioxythiophene):polystyrene sulfonate) coatings that significantly reduce impedance while increasing charge storage capacity. Their electrode designs incorporate nanoscale topographical features that maximize the effective surface area for improved signal transduction. MIT researchers have also developed computational models that accurately predict the electrochemical behavior at the electrode-tissue interface, allowing for optimization of electrode materials and geometries before fabrication. Recent innovations include stretchable, transparent electrodes that maintain electrical performance under mechanical deformation, critical for long-term implantable applications.
Strengths: Superior materials science expertise enabling development of high-performance conductive polymers with excellent biocompatibility; strong interdisciplinary collaboration between electrical engineering, materials science, and neuroscience departments. Weaknesses: Some of their advanced materials solutions may face challenges in scaling to commercial production; regulatory approval pathways for novel electrode materials can be lengthy and complex.
Critical Patents and Research in Bioelectronic Interfaces
Biologically integrated electrode devices
PatentWO2007028003A2
Innovation
- Development of biologically integrated electrode devices with a conductive polymer matrix polymerized in the presence of live tissue, cells, and biological components, enhancing biocompatibility and signal transduction by creating a stable interface with low electrical impedance and increased surface area.
Peripheral Nerve Electrode for Neural Recording and Stimulation
PatentActiveUS20200306527A1
Innovation
- A bioelectric interface with a nerve holder case that restricts the movement of an electrode array to one degree of freedom, allowing precise insertion and stabilization of microelectrode shanks into nerves, minimizing movement-related trauma and foreign body responses, and featuring a sandwiched design between top and bottom parts for enhanced stability and adhesion.
Biocompatibility and Long-term Stability Considerations
Biocompatibility and long-term stability represent critical challenges in bioelectronic interfaces, particularly when considering electrode kinetics. The interaction between electrodes and biological tissues introduces complex requirements that extend beyond mere electrical performance. Materials used in bioelectronic interfaces must demonstrate minimal cytotoxicity while maintaining functional stability in the harsh biological environment characterized by protein adsorption, cellular encapsulation, and varying pH levels.
Recent studies have shown that traditional electrode materials such as platinum and iridium oxide, while offering excellent conductivity, often trigger foreign body responses that compromise long-term functionality. The protein adsorption on electrode surfaces, occurring within minutes of implantation, significantly alters the electrode-tissue interface and consequently affects charge transfer kinetics. This biofouling process typically results in increased impedance and reduced signal quality over time.
Surface modification strategies have emerged as promising approaches to enhance biocompatibility while preserving electrode kinetics. Hydrogel coatings, for instance, provide a biomimetic environment that reduces mechanical mismatch between rigid electrodes and soft tissues. Conductive polymers like PEDOT:PSS offer dual advantages of improved charge transfer capabilities and reduced foreign body response, though their long-term stability remains questionable in chronic applications.
The degradation mechanisms of bioelectronic interfaces present another significant challenge. Oxidative stress induced by redox reactions at the electrode surface can lead to material corrosion and release of potentially toxic byproducts. Studies tracking electrode performance over extended periods (>1 year) have documented gradual increases in impedance and decreases in charge storage capacity, correlating with deteriorating signal quality and therapeutic efficacy.
Accelerated aging tests have become standard practice in evaluating long-term stability, yet these methods often fail to accurately replicate the complex biological environment. The development of more sophisticated in vitro models incorporating dynamic cellular responses and protein interactions represents a current research priority. Additionally, non-invasive imaging techniques for monitoring electrode condition in vivo are advancing, allowing researchers to correlate electrical performance with biological response over time.
Emerging strategies focusing on self-healing materials and biomimetic surface topographies show promise in addressing both biocompatibility and stability concerns. These approaches aim to create dynamic interfaces capable of adapting to biological responses rather than resisting them, potentially revolutionizing the longevity of bioelectronic interfaces and their electrode kinetics in clinical applications.
Recent studies have shown that traditional electrode materials such as platinum and iridium oxide, while offering excellent conductivity, often trigger foreign body responses that compromise long-term functionality. The protein adsorption on electrode surfaces, occurring within minutes of implantation, significantly alters the electrode-tissue interface and consequently affects charge transfer kinetics. This biofouling process typically results in increased impedance and reduced signal quality over time.
Surface modification strategies have emerged as promising approaches to enhance biocompatibility while preserving electrode kinetics. Hydrogel coatings, for instance, provide a biomimetic environment that reduces mechanical mismatch between rigid electrodes and soft tissues. Conductive polymers like PEDOT:PSS offer dual advantages of improved charge transfer capabilities and reduced foreign body response, though their long-term stability remains questionable in chronic applications.
The degradation mechanisms of bioelectronic interfaces present another significant challenge. Oxidative stress induced by redox reactions at the electrode surface can lead to material corrosion and release of potentially toxic byproducts. Studies tracking electrode performance over extended periods (>1 year) have documented gradual increases in impedance and decreases in charge storage capacity, correlating with deteriorating signal quality and therapeutic efficacy.
Accelerated aging tests have become standard practice in evaluating long-term stability, yet these methods often fail to accurately replicate the complex biological environment. The development of more sophisticated in vitro models incorporating dynamic cellular responses and protein interactions represents a current research priority. Additionally, non-invasive imaging techniques for monitoring electrode condition in vivo are advancing, allowing researchers to correlate electrical performance with biological response over time.
Emerging strategies focusing on self-healing materials and biomimetic surface topographies show promise in addressing both biocompatibility and stability concerns. These approaches aim to create dynamic interfaces capable of adapting to biological responses rather than resisting them, potentially revolutionizing the longevity of bioelectronic interfaces and their electrode kinetics in clinical applications.
Regulatory Framework for Implantable Bioelectronic Devices
The regulatory landscape for implantable bioelectronic devices is complex and multifaceted, requiring careful navigation to ensure both patient safety and technological innovation. In the United States, the Food and Drug Administration (FDA) classifies most bioelectronic interfaces as Class III medical devices, necessitating premarket approval (PMA) with comprehensive clinical trials demonstrating safety and efficacy. The electrode kinetics at the tissue-device interface are particularly scrutinized due to their direct impact on biocompatibility and long-term performance.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose similarly stringent requirements, with additional emphasis on post-market surveillance and unique device identification. These regulations specifically address the electrochemical stability of bioelectronic interfaces, requiring manufacturers to demonstrate minimal degradation of electrode materials and consistent charge transfer characteristics over the device's intended lifetime.
International standards such as ISO 14708 (for active implantable medical devices) and IEC 60601 (for medical electrical equipment) provide technical specifications directly relevant to electrode kinetics. These standards define acceptable limits for charge injection capacity, electrode impedance stability, and electrochemical safety margins that must be maintained at the bioelectronic interface to prevent tissue damage or device failure.
Regulatory bodies increasingly require real-time monitoring capabilities for implantable devices, particularly those utilizing novel electrode materials or stimulation paradigms. This trend reflects growing recognition of the dynamic nature of electrode-tissue interactions and the need to detect changes in electrode kinetics that might compromise device performance or patient safety.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have developed specialized pathways for bioelectronic devices, acknowledging their unique risk profiles compared to traditional pharmaceuticals. These frameworks specifically address the characterization of charge transfer mechanisms and faradaic processes at the electrode interface, requiring comprehensive electrochemical profiling before approval.
Emerging regulatory considerations include the development of standards for biodegradable electrodes and adaptive stimulation systems that modify their parameters based on tissue response. These innovations present unique regulatory challenges, as they require frameworks capable of assessing changing electrode kinetics throughout the device lifecycle rather than static performance characteristics.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose similarly stringent requirements, with additional emphasis on post-market surveillance and unique device identification. These regulations specifically address the electrochemical stability of bioelectronic interfaces, requiring manufacturers to demonstrate minimal degradation of electrode materials and consistent charge transfer characteristics over the device's intended lifetime.
International standards such as ISO 14708 (for active implantable medical devices) and IEC 60601 (for medical electrical equipment) provide technical specifications directly relevant to electrode kinetics. These standards define acceptable limits for charge injection capacity, electrode impedance stability, and electrochemical safety margins that must be maintained at the bioelectronic interface to prevent tissue damage or device failure.
Regulatory bodies increasingly require real-time monitoring capabilities for implantable devices, particularly those utilizing novel electrode materials or stimulation paradigms. This trend reflects growing recognition of the dynamic nature of electrode-tissue interactions and the need to detect changes in electrode kinetics that might compromise device performance or patient safety.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have developed specialized pathways for bioelectronic devices, acknowledging their unique risk profiles compared to traditional pharmaceuticals. These frameworks specifically address the characterization of charge transfer mechanisms and faradaic processes at the electrode interface, requiring comprehensive electrochemical profiling before approval.
Emerging regulatory considerations include the development of standards for biodegradable electrodes and adaptive stimulation systems that modify their parameters based on tissue response. These innovations present unique regulatory challenges, as they require frameworks capable of assessing changing electrode kinetics throughout the device lifecycle rather than static performance characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!