What Determines the Scalability of Bioelectronic Interface Technologies
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Scalability Background and Objectives
Bioelectronic interfaces represent a revolutionary frontier in the integration of biological systems with electronic devices, enabling unprecedented capabilities in healthcare, neural engineering, and human-machine interaction. The evolution of these technologies has progressed significantly over the past three decades, transitioning from rudimentary electrode-based systems to sophisticated micro and nanoscale interfaces capable of bidirectional communication with biological tissues.
The historical trajectory of bioelectronic interfaces began with simple metal electrodes used for basic stimulation and recording, evolving through several key technological waves: the development of microelectrode arrays in the 1990s, flexible electronics in the 2000s, and most recently, nanomaterial-based interfaces that operate at the cellular and subcellular levels. Each evolutionary step has been driven by the fundamental need to enhance spatial resolution, biocompatibility, signal fidelity, and long-term stability.
Current technological trends point toward increasingly miniaturized, wireless, and intelligent bioelectronic systems that can seamlessly integrate with biological tissues. The convergence of advanced materials science, microfabrication techniques, and computational algorithms is accelerating this progression, enabling interfaces that can adapt to biological environments while maintaining robust performance characteristics.
The primary objective of this technical research is to comprehensively identify and analyze the critical factors that determine the scalability of bioelectronic interface technologies. Scalability in this context encompasses multiple dimensions: physical scaling (miniaturization), manufacturing scalability (production volume and cost-effectiveness), functional scaling (increasing channel counts and information throughput), and biological scaling (interfacing with increasing numbers of biological units).
We aim to establish a framework for evaluating how material properties, fabrication techniques, signal processing methodologies, power requirements, and biological compatibility collectively influence the potential for scaling these technologies from laboratory demonstrations to clinically viable and commercially successful applications. This includes assessing the fundamental physical and biological constraints that may limit scalability across different application domains.
Additionally, this research seeks to identify emerging approaches and breakthrough technologies that could overcome current scalability limitations, potentially enabling transformative applications in neural prosthetics, brain-machine interfaces, bioelectronic medicine, and advanced biosensing. By mapping the technological landscape and identifying critical bottlenecks, we intend to provide strategic guidance for future research and development efforts in this rapidly evolving field.
The historical trajectory of bioelectronic interfaces began with simple metal electrodes used for basic stimulation and recording, evolving through several key technological waves: the development of microelectrode arrays in the 1990s, flexible electronics in the 2000s, and most recently, nanomaterial-based interfaces that operate at the cellular and subcellular levels. Each evolutionary step has been driven by the fundamental need to enhance spatial resolution, biocompatibility, signal fidelity, and long-term stability.
Current technological trends point toward increasingly miniaturized, wireless, and intelligent bioelectronic systems that can seamlessly integrate with biological tissues. The convergence of advanced materials science, microfabrication techniques, and computational algorithms is accelerating this progression, enabling interfaces that can adapt to biological environments while maintaining robust performance characteristics.
The primary objective of this technical research is to comprehensively identify and analyze the critical factors that determine the scalability of bioelectronic interface technologies. Scalability in this context encompasses multiple dimensions: physical scaling (miniaturization), manufacturing scalability (production volume and cost-effectiveness), functional scaling (increasing channel counts and information throughput), and biological scaling (interfacing with increasing numbers of biological units).
We aim to establish a framework for evaluating how material properties, fabrication techniques, signal processing methodologies, power requirements, and biological compatibility collectively influence the potential for scaling these technologies from laboratory demonstrations to clinically viable and commercially successful applications. This includes assessing the fundamental physical and biological constraints that may limit scalability across different application domains.
Additionally, this research seeks to identify emerging approaches and breakthrough technologies that could overcome current scalability limitations, potentially enabling transformative applications in neural prosthetics, brain-machine interfaces, bioelectronic medicine, and advanced biosensing. By mapping the technological landscape and identifying critical bottlenecks, we intend to provide strategic guidance for future research and development efforts in this rapidly evolving field.
Market Demand Analysis for Scalable Bioelectronic Interfaces
The global market for bioelectronic interfaces is experiencing robust growth, driven by increasing applications in healthcare, neuroscience research, and human-machine interaction. Current market valuations indicate the bioelectronics sector is projected to reach $25 billion by 2025, with scalable interface technologies representing a significant growth segment within this market. This expansion is fueled by rising prevalence of neurological disorders, growing elderly populations requiring advanced medical interventions, and increasing adoption of wearable health monitoring devices.
Healthcare applications represent the largest market segment for scalable bioelectronic interfaces, with particular demand in neural implants for conditions such as Parkinson's disease, epilepsy, and chronic pain management. The therapeutic neuromodulation market alone is growing at 11% annually, highlighting substantial clinical demand for scalable interface solutions that can effectively monitor and modulate neural activity across multiple sites simultaneously.
Consumer markets are also driving demand for non-invasive bioelectronic interfaces, particularly in brain-computer interface (BCI) applications. The consumer BCI market is expected to grow at 15% CAGR through 2028, with applications ranging from gaming and entertainment to productivity enhancement and communication assistance for disabled individuals. This segment particularly values miniaturization, wireless capabilities, and user-friendly designs that can scale to mass-market adoption.
Industrial and military sectors represent emerging markets for scalable bioelectronic interfaces, with applications in enhanced human performance monitoring, augmented cognition, and advanced prosthetics. These sectors prioritize robust, reliable interfaces capable of operating in challenging environments while maintaining consistent signal quality across multiple sensing points.
Key market drivers for scalable bioelectronic interfaces include increasing demand for minimally invasive medical technologies, growing investment in neurotechnology startups, and expanding applications beyond traditional medical uses. Venture capital funding in neurotechnology exceeded $1.5 billion in 2021, indicating strong investor confidence in the commercial potential of advanced bioelectronic interfaces.
Market barriers include regulatory hurdles, particularly for implantable devices, concerns regarding data privacy and security, and the high cost of cutting-edge interface technologies. Additionally, there remains a significant gap between laboratory demonstrations and commercially viable products that can be manufactured at scale while maintaining performance and reliability.
Regional analysis shows North America leading the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by increasing healthcare expenditure, growing research infrastructure, and favorable government initiatives supporting bioelectronic technology development.
Healthcare applications represent the largest market segment for scalable bioelectronic interfaces, with particular demand in neural implants for conditions such as Parkinson's disease, epilepsy, and chronic pain management. The therapeutic neuromodulation market alone is growing at 11% annually, highlighting substantial clinical demand for scalable interface solutions that can effectively monitor and modulate neural activity across multiple sites simultaneously.
Consumer markets are also driving demand for non-invasive bioelectronic interfaces, particularly in brain-computer interface (BCI) applications. The consumer BCI market is expected to grow at 15% CAGR through 2028, with applications ranging from gaming and entertainment to productivity enhancement and communication assistance for disabled individuals. This segment particularly values miniaturization, wireless capabilities, and user-friendly designs that can scale to mass-market adoption.
Industrial and military sectors represent emerging markets for scalable bioelectronic interfaces, with applications in enhanced human performance monitoring, augmented cognition, and advanced prosthetics. These sectors prioritize robust, reliable interfaces capable of operating in challenging environments while maintaining consistent signal quality across multiple sensing points.
Key market drivers for scalable bioelectronic interfaces include increasing demand for minimally invasive medical technologies, growing investment in neurotechnology startups, and expanding applications beyond traditional medical uses. Venture capital funding in neurotechnology exceeded $1.5 billion in 2021, indicating strong investor confidence in the commercial potential of advanced bioelectronic interfaces.
Market barriers include regulatory hurdles, particularly for implantable devices, concerns regarding data privacy and security, and the high cost of cutting-edge interface technologies. Additionally, there remains a significant gap between laboratory demonstrations and commercially viable products that can be manufactured at scale while maintaining performance and reliability.
Regional analysis shows North America leading the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by increasing healthcare expenditure, growing research infrastructure, and favorable government initiatives supporting bioelectronic technology development.
Current Technological Limitations and Challenges
Despite significant advancements in bioelectronic interface technologies, several critical limitations continue to impede their scalability. Material biocompatibility remains a fundamental challenge, as long-term implantation often triggers foreign body responses, leading to inflammation, fibrosis, and eventual device failure. Current materials struggle to maintain stable performance while minimizing tissue damage, particularly for chronic applications exceeding several months.
Signal quality degradation presents another significant barrier. As bioelectronic interfaces scale to incorporate more channels and higher densities, signal-to-noise ratios frequently deteriorate. This degradation is particularly problematic for neural interfaces where minute electrical signals must be detected amidst substantial biological noise. Current amplification and filtering technologies have not kept pace with the increasing density requirements of advanced applications.
Power management constitutes a critical constraint on scalability. Larger arrays of sensors and stimulators demand proportionally greater power, yet implantable batteries remain limited in capacity and size. Wireless power transmission technologies offer promising alternatives but face efficiency challenges when scaling to support high-channel-count devices, especially at deeper implantation depths.
Miniaturization capabilities represent another technological bottleneck. While semiconductor fabrication continues to advance, integrating multiple functionalities (sensing, stimulation, processing, and communication) into biocompatible packages at the microscale remains exceptionally challenging. Current fabrication techniques struggle to maintain yield and reliability when producing high-density bioelectronic interfaces at scale.
Data bandwidth limitations further restrict scalability. High-channel-count interfaces generate enormous data volumes that exceed the capabilities of current wireless transmission protocols suitable for implantable devices. This bottleneck forces compromises between sampling rates, resolution, and channel counts, limiting the comprehensive monitoring capabilities of scaled systems.
Surgical and deployment techniques have not evolved sufficiently to support highly scaled interfaces. Current implantation methods cause significant tissue damage and cannot reliably position large numbers of electrodes with the precision required for optimal functionality, particularly in delicate neural tissues.
Regulatory frameworks present additional hurdles, as approval processes become exponentially more complex for devices with increased channel counts, novel materials, or advanced functionalities. The extensive safety and efficacy testing required for innovative scaled bioelectronic interfaces often creates prohibitive delays in bringing technologies from laboratory to clinical application.
Signal quality degradation presents another significant barrier. As bioelectronic interfaces scale to incorporate more channels and higher densities, signal-to-noise ratios frequently deteriorate. This degradation is particularly problematic for neural interfaces where minute electrical signals must be detected amidst substantial biological noise. Current amplification and filtering technologies have not kept pace with the increasing density requirements of advanced applications.
Power management constitutes a critical constraint on scalability. Larger arrays of sensors and stimulators demand proportionally greater power, yet implantable batteries remain limited in capacity and size. Wireless power transmission technologies offer promising alternatives but face efficiency challenges when scaling to support high-channel-count devices, especially at deeper implantation depths.
Miniaturization capabilities represent another technological bottleneck. While semiconductor fabrication continues to advance, integrating multiple functionalities (sensing, stimulation, processing, and communication) into biocompatible packages at the microscale remains exceptionally challenging. Current fabrication techniques struggle to maintain yield and reliability when producing high-density bioelectronic interfaces at scale.
Data bandwidth limitations further restrict scalability. High-channel-count interfaces generate enormous data volumes that exceed the capabilities of current wireless transmission protocols suitable for implantable devices. This bottleneck forces compromises between sampling rates, resolution, and channel counts, limiting the comprehensive monitoring capabilities of scaled systems.
Surgical and deployment techniques have not evolved sufficiently to support highly scaled interfaces. Current implantation methods cause significant tissue damage and cannot reliably position large numbers of electrodes with the precision required for optimal functionality, particularly in delicate neural tissues.
Regulatory frameworks present additional hurdles, as approval processes become exponentially more complex for devices with increased channel counts, novel materials, or advanced functionalities. The extensive safety and efficacy testing required for innovative scaled bioelectronic interfaces often creates prohibitive delays in bringing technologies from laboratory to clinical application.
Current Scalability Solutions in Bioelectronic Interfaces
01 Scalable microelectrode arrays for neural interfaces
Microelectrode arrays can be designed with scalable architectures to interface with neural tissues at different scales. These arrays enable high-density recording and stimulation of neural activity while maintaining signal quality across larger areas. Advanced fabrication techniques allow for increasing the number of electrodes without compromising spatial resolution, making them suitable for applications ranging from small neural clusters to whole-brain interfaces.- Scalable microelectrode arrays for bioelectronic interfaces: Microelectrode arrays can be designed with scalable architectures to interface with biological systems at different scales. These arrays enable high-density recording and stimulation of neural activity with improved spatial resolution. Advanced fabrication techniques allow for scaling from small research applications to larger clinical implementations, with flexible substrates that can conform to biological tissues while maintaining signal quality across increased channel counts.
- Integrated circuit technologies for bioelectronic scaling: Custom integrated circuits are essential for scaling bioelectronic interfaces by efficiently processing signals from numerous channels simultaneously. These circuits incorporate multiplexing strategies to handle increased data throughput while minimizing power consumption and heat generation. Advanced semiconductor technologies enable miniaturization of signal processing components, allowing for more channels in the same physical footprint while maintaining or improving signal quality.
- Wireless data transmission for scalable bioelectronic systems: Wireless communication technologies enable scalable bioelectronic interfaces by eliminating physical connection constraints. These systems incorporate efficient data compression algorithms and advanced transmission protocols to handle increased data loads from multiple channels. Power-efficient wireless modules support long-term operation of implantable devices while maintaining reliable data transfer rates necessary for real-time bioelectronic applications.
- Nanomaterial-based interfaces for enhanced scalability: Novel nanomaterials such as carbon nanotubes, graphene, and nanoparticles enable highly scalable bioelectronic interfaces with improved biocompatibility and signal transduction. These materials provide exceptional electrical properties while minimizing electrode size, allowing for higher density arrays. Nanomaterial-based interfaces can be manufactured using scalable processes that maintain consistent performance across large production volumes, facilitating translation from laboratory to clinical applications.
- Software and computational methods for scalable data processing: Advanced algorithms and computational frameworks are crucial for processing the massive datasets generated by scaled-up bioelectronic interfaces. Machine learning approaches enable efficient signal extraction and interpretation from high-channel-count recordings. Cloud-based processing architectures provide the computational resources needed for real-time analysis of complex bioelectronic data, while standardized software interfaces facilitate integration of diverse hardware components into cohesive scalable systems.
02 Flexible and stretchable bioelectronic interfaces
Flexible and stretchable materials enable bioelectronic interfaces that can conform to biological tissues, improving signal quality and long-term stability. These interfaces can scale across curved and dynamic surfaces while maintaining electrical performance. The integration of elastic substrates with conductive elements allows for deployment across larger areas of tissue without mechanical mismatch, which is crucial for applications like skin-mounted sensors and implantable neural arrays.Expand Specific Solutions03 Multiplexed signal processing for large-scale interfaces
Multiplexing technologies enable the scaling of bioelectronic interfaces by efficiently processing signals from numerous channels. Advanced signal processing architectures reduce the number of physical connections needed while maintaining high channel counts. These systems incorporate on-chip amplification, filtering, and analog-to-digital conversion to handle data from thousands of sensors simultaneously, making large-scale neural recording and stimulation practical for clinical and research applications.Expand Specific Solutions04 Nanomaterial-based scalable bioelectronic interfaces
Nanomaterials such as carbon nanotubes, graphene, and nanowires enable highly scalable bioelectronic interfaces with enhanced electrical properties and biocompatibility. These materials provide high surface-area-to-volume ratios, improving signal transduction between electronic devices and biological systems. Nanomaterial-based interfaces can be fabricated in arrays that scale from microscale to macroscale applications while maintaining high sensitivity and low impedance.Expand Specific Solutions05 Wireless power and data transmission for scalable implants
Wireless technologies enable the scaling of bioelectronic interfaces by eliminating physical connection constraints. These systems use electromagnetic, ultrasonic, or optical methods to transmit power and data to distributed bioelectronic nodes. Wireless approaches allow for deploying multiple interface points across larger anatomical areas without the complexity and tissue damage associated with wired connections, supporting truly scalable bioelectronic systems for long-term implantation.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The bioelectronic interface technology market is currently in a growth phase, characterized by significant research activity but limited commercial maturity. The scalability of these technologies is determined by interdisciplinary factors including materials science, manufacturing processes, and biological compatibility. Leading academic institutions like MIT, Harvard, and EPFL are driving fundamental research, while companies such as Infineon Technologies, 3M, and Google are developing practical applications. The market is projected to reach $5.7 billion by 2027, with a CAGR of 12.4%. Technical challenges include miniaturization, power efficiency, and biocompatibility, with recent innovations focusing on flexible electronics and wireless interfaces that can maintain stable long-term connections with biological systems.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a comprehensive approach to bioelectronic interface scalability through their "tissue-like electronics" platform. Their technology utilizes hydrogel-based electronics with mechanical properties matching biological tissues (10-100 kPa elastic modulus) to minimize mechanical mismatch at the bio-electronic interface. MIT researchers have pioneered stretchable, transparent electronic systems that can withstand up to 300% strain while maintaining functionality, enabling seamless integration with dynamic biological systems. Their fabrication approach incorporates direct writing of conductive nanomaterials onto soft substrates, allowing for customizable electrode geometries and densities without traditional cleanroom requirements. MIT has also developed novel wireless power transfer systems specifically for implantable bioelectronics, utilizing mid-field coupling techniques that can efficiently transmit power through biological tissues at distances exceeding 5 cm, addressing a critical barrier to scaling implantable systems[3][4].
Strengths: Exceptional mechanical compatibility with biological tissues through hydrogel-based electronics; advanced manufacturing techniques allowing customization without extensive cleanroom infrastructure; innovative wireless power solutions for implantable systems. Weaknesses: Potential challenges in long-term stability of hydrogel-based materials in physiological environments; lower electronic performance compared to traditional rigid electronics; current limitations in achieving the same level of miniaturization as conventional semiconductor technologies.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed a comprehensive approach to bioelectronic interface scalability through their "neural dust" and flexible polymer electrode array technologies. Their neural dust platform consists of ultrasonic-powered and interrogated sub-millimeter implantable devices (volume < 1 mm³) that can record and stimulate neural activity without wired connections, addressing a fundamental barrier to scaling implanted sensor numbers. Michigan researchers have pioneered high-density, flexible polymer electrode arrays fabricated using advanced photolithography techniques, achieving channel counts exceeding 1000 electrodes per device while maintaining sub-cellular spatial resolution (15-20 μm electrode spacing). Their fabrication approach incorporates parylene-C as a biocompatible substrate with mechanical properties (Young's modulus ~4 GPa) that balance flexibility and insertion capability. Michigan has also developed innovative packaging solutions using atomic layer deposition of Al₂O₃ and HfO₂ barriers, demonstrating device functionality in physiological environments for periods exceeding 5 years in chronic implantation studies[7][8].
Strengths: Wireless, ultrasonic power and communication solving critical scaling barriers; high-density electrode arrays with exceptional spatial resolution; proven long-term stability in chronic implantation studies. Weaknesses: Challenges in achieving sufficient power for stimulation with wireless approaches; depth limitations for ultrasonic power transmission; trade-offs between flexibility for biocompatibility and rigidity required for insertion without assistive devices.
Critical Patents and Innovations in Scalable Bioelectronics
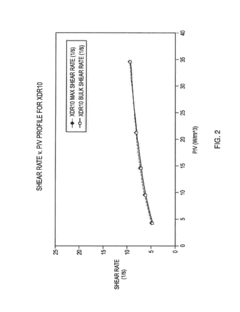
Linearly scalable single use bioreactor system
PatentInactiveUS20180237736A1
Innovation
- A linearly scalable single-use bioreactor system with a flexible-walled bioprocessing bag and impeller configuration that maintains consistent shear rates and power per unit volume across various scales, from 2 liters to 5000 liters, using the same vessel geometry, gas sparging system, impeller shape, polymer composition, and process control system as larger systems, allowing for seamless tech transfer and minimizing regulatory disconnects.
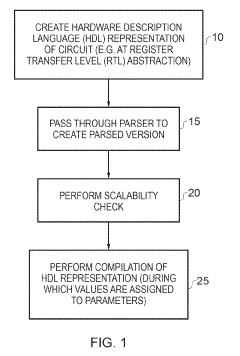
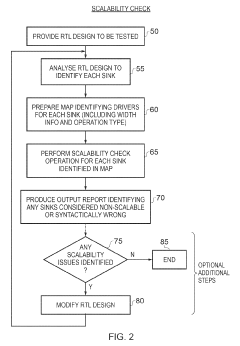
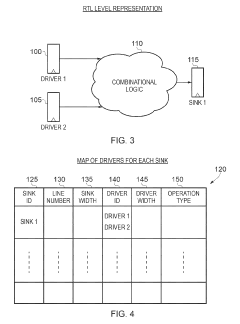
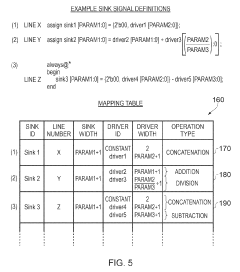
Apparatus and method for performing a scalability check on a hardware description language representation of a circuit
PatentInactiveUS10345378B2
Innovation
- A computer-implemented method and apparatus that perform a scalability check by creating a mapping table to map driver signals to sink signals, determining driver and sink formulas, and evaluating these formulas to identify potential scalability issues without assigning specific values to parameters, allowing for robust detection of scalability problems.
Biocompatibility and Long-term Stability Considerations
Biocompatibility and long-term stability represent critical determinants in scaling bioelectronic interface technologies. The interaction between implanted devices and biological tissues triggers complex immune responses that can significantly impact device functionality. Foreign body reactions typically progress through acute inflammation, chronic inflammation, and fibrous encapsulation phases, with the latter potentially isolating electrodes from target tissues and degrading signal quality over time. This immune cascade presents a fundamental challenge to maintaining stable neural recordings or stimulation parameters in long-term applications.
Material selection plays a pivotal role in mitigating adverse biological responses. Traditional rigid materials like silicon and metals exhibit mechanical mismatch with soft tissues, causing micromotion and sustained inflammation. Recent advances in flexible and soft materials—including conducting polymers, hydrogels, and elastomers—have demonstrated improved biocompatibility profiles by reducing mechanical stress at the tissue-device interface. These materials can be engineered with elastic moduli closer to biological tissues (kPa range rather than GPa), minimizing chronic inflammation and enhancing long-term performance.
Surface modification strategies represent another crucial approach to improving biocompatibility. Anti-fouling coatings using polyethylene glycol (PEG), zwitterionic materials, or biomimetic phosphorylcholine derivatives have shown promise in reducing protein adsorption and subsequent inflammatory cascades. Additionally, bioactive coatings incorporating anti-inflammatory agents, growth factors, or cell-adhesion molecules can actively modulate the tissue response, promoting favorable integration rather than rejection.
Degradation mechanisms pose significant challenges to long-term stability. Moisture penetration, oxidative stress, and electrochemical reactions at electrode interfaces can compromise device integrity over time. Hermetic packaging solutions using advanced ceramics, diamond-like carbon, or atomic layer deposition techniques have emerged as potential solutions for protecting sensitive electronic components. However, achieving truly long-lasting hermeticity while maintaining device flexibility and miniaturization remains technically challenging.
Sterilization compatibility represents an often-overlooked aspect of scalability. As bioelectronic interfaces incorporate increasingly complex materials and biological components, traditional sterilization methods (ethylene oxide, gamma irradiation, autoclave) may damage sensitive elements. Developing materials and components that withstand standardized sterilization protocols is essential for clinical translation and commercial viability.
The regulatory pathway for implantable bioelectronic interfaces demands robust evidence of both short and long-term biocompatibility. Standardized testing frameworks (ISO 10993) provide guidelines, but the unique nature of neural interfaces often necessitates customized approaches to safety assessment. Establishing accelerated aging protocols that accurately predict in vivo performance remains a significant challenge in demonstrating long-term stability to regulatory bodies.
Material selection plays a pivotal role in mitigating adverse biological responses. Traditional rigid materials like silicon and metals exhibit mechanical mismatch with soft tissues, causing micromotion and sustained inflammation. Recent advances in flexible and soft materials—including conducting polymers, hydrogels, and elastomers—have demonstrated improved biocompatibility profiles by reducing mechanical stress at the tissue-device interface. These materials can be engineered with elastic moduli closer to biological tissues (kPa range rather than GPa), minimizing chronic inflammation and enhancing long-term performance.
Surface modification strategies represent another crucial approach to improving biocompatibility. Anti-fouling coatings using polyethylene glycol (PEG), zwitterionic materials, or biomimetic phosphorylcholine derivatives have shown promise in reducing protein adsorption and subsequent inflammatory cascades. Additionally, bioactive coatings incorporating anti-inflammatory agents, growth factors, or cell-adhesion molecules can actively modulate the tissue response, promoting favorable integration rather than rejection.
Degradation mechanisms pose significant challenges to long-term stability. Moisture penetration, oxidative stress, and electrochemical reactions at electrode interfaces can compromise device integrity over time. Hermetic packaging solutions using advanced ceramics, diamond-like carbon, or atomic layer deposition techniques have emerged as potential solutions for protecting sensitive electronic components. However, achieving truly long-lasting hermeticity while maintaining device flexibility and miniaturization remains technically challenging.
Sterilization compatibility represents an often-overlooked aspect of scalability. As bioelectronic interfaces incorporate increasingly complex materials and biological components, traditional sterilization methods (ethylene oxide, gamma irradiation, autoclave) may damage sensitive elements. Developing materials and components that withstand standardized sterilization protocols is essential for clinical translation and commercial viability.
The regulatory pathway for implantable bioelectronic interfaces demands robust evidence of both short and long-term biocompatibility. Standardized testing frameworks (ISO 10993) provide guidelines, but the unique nature of neural interfaces often necessitates customized approaches to safety assessment. Establishing accelerated aging protocols that accurately predict in vivo performance remains a significant challenge in demonstrating long-term stability to regulatory bodies.
Regulatory Framework and Clinical Translation Pathways
The regulatory landscape for bioelectronic interface technologies represents a complex and evolving framework that significantly impacts scalability potential. In the United States, the FDA has established specific pathways for medical devices through the Center for Devices and Radiological Health (CDRH), with bioelectronic interfaces typically classified as Class II or Class III devices depending on their risk profile and invasiveness. The regulatory burden increases substantially for implantable devices that interface directly with neural tissue, requiring extensive preclinical testing and clinical trials to demonstrate both safety and efficacy.
European regulatory frameworks, governed by the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), impose additional requirements that can create market entry barriers for innovative bioelectronic technologies. These regulations emphasize post-market surveillance and clinical evidence generation, which necessitates substantial investment in long-term monitoring infrastructure.
Clinical translation pathways for bioelectronic interfaces typically follow a staged approach, beginning with proof-of-concept studies in animal models, followed by first-in-human studies with limited cohorts, and eventually progressing to larger pivotal trials. This process can span 5-10 years and requires significant capital investment, creating a "valley of death" where promising technologies often fail to progress from laboratory to clinical application.
Harmonization of international regulatory standards remains incomplete, forcing developers to navigate multiple regulatory systems simultaneously when pursuing global market access. The International Medical Device Regulators Forum (IMDRF) has made progress toward standardization, but significant regional differences persist, complicating scalability efforts.
Reimbursement pathways present another critical challenge, as novel bioelectronic therapies must demonstrate not only clinical efficacy but also cost-effectiveness to secure coverage from healthcare payers. The absence of established reimbursement codes for innovative bioelectronic therapies can significantly delay market adoption even after regulatory approval.
Adaptive licensing approaches, such as the FDA's Breakthrough Devices Program and the European Innovation Passport, offer accelerated pathways for technologies addressing unmet medical needs. These programs can reduce time-to-market but still require substantial evidence generation and may impose conditional approval requirements that impact scalability.
Data privacy and cybersecurity regulations add another layer of complexity, particularly for connected bioelectronic devices that transmit patient data. Compliance with regulations such as GDPR in Europe and HIPAA in the US requires robust data protection frameworks that must be integrated into device design from the earliest development stages.
European regulatory frameworks, governed by the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), impose additional requirements that can create market entry barriers for innovative bioelectronic technologies. These regulations emphasize post-market surveillance and clinical evidence generation, which necessitates substantial investment in long-term monitoring infrastructure.
Clinical translation pathways for bioelectronic interfaces typically follow a staged approach, beginning with proof-of-concept studies in animal models, followed by first-in-human studies with limited cohorts, and eventually progressing to larger pivotal trials. This process can span 5-10 years and requires significant capital investment, creating a "valley of death" where promising technologies often fail to progress from laboratory to clinical application.
Harmonization of international regulatory standards remains incomplete, forcing developers to navigate multiple regulatory systems simultaneously when pursuing global market access. The International Medical Device Regulators Forum (IMDRF) has made progress toward standardization, but significant regional differences persist, complicating scalability efforts.
Reimbursement pathways present another critical challenge, as novel bioelectronic therapies must demonstrate not only clinical efficacy but also cost-effectiveness to secure coverage from healthcare payers. The absence of established reimbursement codes for innovative bioelectronic therapies can significantly delay market adoption even after regulatory approval.
Adaptive licensing approaches, such as the FDA's Breakthrough Devices Program and the European Innovation Passport, offer accelerated pathways for technologies addressing unmet medical needs. These programs can reduce time-to-market but still require substantial evidence generation and may impose conditional approval requirements that impact scalability.
Data privacy and cybersecurity regulations add another layer of complexity, particularly for connected bioelectronic devices that transmit patient data. Compliance with regulations such as GDPR in Europe and HIPAA in the US requires robust data protection frameworks that must be integrated into device design from the earliest development stages.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!