What Role Does Bioelectronic Interface Play in Anti-Counterfeiting Technologies
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectronic Interface Anti-Counterfeiting Background and Objectives
Bioelectronic interfaces represent a convergence of biological systems with electronic components, creating a revolutionary approach to anti-counterfeiting technologies. This field has evolved significantly over the past decade, transitioning from simple electronic tags to sophisticated bioelectronic systems that leverage unique biological signatures. The integration of biological elements with electronic detection mechanisms has created authentication solutions that are inherently difficult to replicate due to their complexity and biological specificity.
The evolution of anti-counterfeiting technologies has historically progressed from physical features (watermarks, holograms) to digital solutions (QR codes, RFID), and now to bioelectronic interfaces that combine biological markers with electronic detection systems. This progression reflects the continuous arms race between security developers and counterfeiters, necessitating increasingly sophisticated authentication methods.
Bioelectronic interfaces in anti-counterfeiting applications typically involve biological components such as DNA sequences, proteins, enzymes, or living cells integrated with electronic sensing platforms. These systems can detect specific biological interactions and translate them into electronic signals, providing a unique verification mechanism that is extremely difficult to reverse-engineer or duplicate without advanced knowledge and equipment.
The primary objective of bioelectronic interface development in anti-counterfeiting is to create authentication systems that offer unprecedented levels of security while remaining cost-effective and practical for widespread implementation. These technologies aim to protect high-value products in pharmaceuticals, luxury goods, electronics, and official documents from counterfeiting, which causes significant economic damage estimated at over $500 billion annually worldwide.
Current technical goals include miniaturization of bioelectronic components, extending shelf life of biological elements, reducing manufacturing costs, and developing user-friendly detection systems that don't require specialized training or equipment. Researchers are also focusing on creating bioelectronic interfaces that can be seamlessly integrated into existing manufacturing processes without significant disruption.
The future trajectory of bioelectronic anti-counterfeiting technologies points toward self-powered systems, remote authentication capabilities, and multi-factor bioelectronic verification methods. These advancements aim to create a new generation of anti-counterfeiting solutions that not only verify product authenticity but also track product lifecycle, monitor storage conditions, and potentially even detect tampering attempts in real-time.
As global supply chains become increasingly complex and counterfeiting techniques more sophisticated, bioelectronic interfaces represent a promising frontier in securing product authenticity and protecting both consumers and brand integrity in the global marketplace.
The evolution of anti-counterfeiting technologies has historically progressed from physical features (watermarks, holograms) to digital solutions (QR codes, RFID), and now to bioelectronic interfaces that combine biological markers with electronic detection systems. This progression reflects the continuous arms race between security developers and counterfeiters, necessitating increasingly sophisticated authentication methods.
Bioelectronic interfaces in anti-counterfeiting applications typically involve biological components such as DNA sequences, proteins, enzymes, or living cells integrated with electronic sensing platforms. These systems can detect specific biological interactions and translate them into electronic signals, providing a unique verification mechanism that is extremely difficult to reverse-engineer or duplicate without advanced knowledge and equipment.
The primary objective of bioelectronic interface development in anti-counterfeiting is to create authentication systems that offer unprecedented levels of security while remaining cost-effective and practical for widespread implementation. These technologies aim to protect high-value products in pharmaceuticals, luxury goods, electronics, and official documents from counterfeiting, which causes significant economic damage estimated at over $500 billion annually worldwide.
Current technical goals include miniaturization of bioelectronic components, extending shelf life of biological elements, reducing manufacturing costs, and developing user-friendly detection systems that don't require specialized training or equipment. Researchers are also focusing on creating bioelectronic interfaces that can be seamlessly integrated into existing manufacturing processes without significant disruption.
The future trajectory of bioelectronic anti-counterfeiting technologies points toward self-powered systems, remote authentication capabilities, and multi-factor bioelectronic verification methods. These advancements aim to create a new generation of anti-counterfeiting solutions that not only verify product authenticity but also track product lifecycle, monitor storage conditions, and potentially even detect tampering attempts in real-time.
As global supply chains become increasingly complex and counterfeiting techniques more sophisticated, bioelectronic interfaces represent a promising frontier in securing product authenticity and protecting both consumers and brand integrity in the global marketplace.
Market Demand Analysis for Bioelectronic Authentication
The global market for anti-counterfeiting technologies has experienced significant growth in recent years, driven by increasing concerns about product authenticity and security across various industries. The bioelectronic authentication segment specifically has emerged as a promising frontier, with market analysts projecting substantial expansion over the next decade.
The pharmaceutical industry represents the largest demand sector for bioelectronic authentication solutions, accounting for approximately one-third of the total market share. This demand stems from the critical need to protect patients from counterfeit medications, which not only pose serious health risks but also result in significant revenue losses for pharmaceutical companies. According to industry reports, counterfeit pharmaceuticals cause annual losses exceeding billions of dollars globally.
Consumer electronics manufacturers have also become major adopters of bioelectronic authentication technologies, particularly for high-value devices and components. The integration of bioelectronic interfaces in smartphones, tablets, and wearable devices has created a substantial market segment with strong growth potential.
Luxury goods producers represent another significant market, with premium brands increasingly implementing sophisticated bioelectronic authentication measures to protect their products and brand equity. The fashion, jewelry, and accessories sectors have shown particular interest in bioelectronic solutions that can be seamlessly integrated into their products without compromising aesthetics.
Government and security sectors constitute a rapidly growing market segment, with applications ranging from secure identification documents to currency protection. The adoption of bioelectronic interfaces in passports, identity cards, and banknotes has accelerated in response to increasingly sophisticated counterfeiting techniques.
Regional analysis reveals that North America currently leads the bioelectronic authentication market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by increasing manufacturing activities, rising disposable incomes, and growing awareness about product authenticity.
Consumer awareness and willingness to pay for authentic products have significantly influenced market dynamics. Recent surveys indicate that consumers are becoming more concerned about product authenticity and are increasingly willing to utilize authentication technologies, including bioelectronic interfaces, to verify purchases.
The COVID-19 pandemic has accelerated market growth by highlighting vulnerabilities in supply chains and increasing concerns about counterfeit medical supplies and pharmaceuticals. This has prompted many organizations to reevaluate and strengthen their anti-counterfeiting measures, creating additional opportunities for bioelectronic authentication solutions.
The pharmaceutical industry represents the largest demand sector for bioelectronic authentication solutions, accounting for approximately one-third of the total market share. This demand stems from the critical need to protect patients from counterfeit medications, which not only pose serious health risks but also result in significant revenue losses for pharmaceutical companies. According to industry reports, counterfeit pharmaceuticals cause annual losses exceeding billions of dollars globally.
Consumer electronics manufacturers have also become major adopters of bioelectronic authentication technologies, particularly for high-value devices and components. The integration of bioelectronic interfaces in smartphones, tablets, and wearable devices has created a substantial market segment with strong growth potential.
Luxury goods producers represent another significant market, with premium brands increasingly implementing sophisticated bioelectronic authentication measures to protect their products and brand equity. The fashion, jewelry, and accessories sectors have shown particular interest in bioelectronic solutions that can be seamlessly integrated into their products without compromising aesthetics.
Government and security sectors constitute a rapidly growing market segment, with applications ranging from secure identification documents to currency protection. The adoption of bioelectronic interfaces in passports, identity cards, and banknotes has accelerated in response to increasingly sophisticated counterfeiting techniques.
Regional analysis reveals that North America currently leads the bioelectronic authentication market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by increasing manufacturing activities, rising disposable incomes, and growing awareness about product authenticity.
Consumer awareness and willingness to pay for authentic products have significantly influenced market dynamics. Recent surveys indicate that consumers are becoming more concerned about product authenticity and are increasingly willing to utilize authentication technologies, including bioelectronic interfaces, to verify purchases.
The COVID-19 pandemic has accelerated market growth by highlighting vulnerabilities in supply chains and increasing concerns about counterfeit medical supplies and pharmaceuticals. This has prompted many organizations to reevaluate and strengthen their anti-counterfeiting measures, creating additional opportunities for bioelectronic authentication solutions.
Current State and Challenges in Bioelectronic Anti-Counterfeiting
Bioelectronic interfaces for anti-counterfeiting technologies currently exist at the intersection of multiple disciplines, including materials science, electronics, biology, and information security. The global landscape shows significant advancements in developed economies like the United States, European Union, Japan, and increasingly China, where research institutions and technology companies are actively developing sophisticated bioelectronic authentication systems.
The current state of bioelectronic anti-counterfeiting technology can be categorized into several key approaches. DNA-based authentication systems utilize unique genetic sequences as security features, offering extremely high levels of security but facing challenges in field detection speed and cost-effectiveness. Protein-based recognition systems leverage antibody-antigen interactions for verification, providing good specificity but struggling with environmental stability issues.
Enzyme-based reactive systems that produce visible color changes upon authentication represent another promising direction, though they often require careful handling to maintain enzymatic activity. Cell-based biosensors that respond to specific stimuli offer dynamic authentication capabilities but face significant hurdles in maintaining cell viability during product lifecycle.
The integration of these biological elements with electronic components creates hybrid systems capable of both storing authentication information and transmitting verification data. Recent innovations include bioelectronic tags that combine biological markers with RFID or NFC technology, enabling seamless authentication through standard mobile devices.
Despite these advancements, significant challenges persist. Stability remains a primary concern, as biological components are inherently sensitive to environmental conditions including temperature, humidity, and light exposure. This vulnerability often necessitates additional protective measures that increase complexity and cost. Scalability presents another major hurdle, as many current bioelectronic solutions require specialized manufacturing processes that are difficult to implement at industrial scales.
Standardization across the industry is notably lacking, with different proprietary systems using incompatible verification methods. This fragmentation impedes widespread adoption and increases implementation costs. Detection reliability also remains problematic, with some systems showing unacceptable false positive or false negative rates in field conditions.
Regulatory frameworks for bioelectronic authentication technologies are still evolving, creating uncertainty for manufacturers and implementers. Concerns regarding potential environmental impacts of bioelectronic tags, particularly those containing genetically modified materials, have also emerged as adoption increases.
Cost considerations represent perhaps the most significant barrier to widespread implementation. Current bioelectronic anti-counterfeiting solutions typically cost substantially more than conventional methods, limiting their application to high-value products where the authentication premium can be justified.
The current state of bioelectronic anti-counterfeiting technology can be categorized into several key approaches. DNA-based authentication systems utilize unique genetic sequences as security features, offering extremely high levels of security but facing challenges in field detection speed and cost-effectiveness. Protein-based recognition systems leverage antibody-antigen interactions for verification, providing good specificity but struggling with environmental stability issues.
Enzyme-based reactive systems that produce visible color changes upon authentication represent another promising direction, though they often require careful handling to maintain enzymatic activity. Cell-based biosensors that respond to specific stimuli offer dynamic authentication capabilities but face significant hurdles in maintaining cell viability during product lifecycle.
The integration of these biological elements with electronic components creates hybrid systems capable of both storing authentication information and transmitting verification data. Recent innovations include bioelectronic tags that combine biological markers with RFID or NFC technology, enabling seamless authentication through standard mobile devices.
Despite these advancements, significant challenges persist. Stability remains a primary concern, as biological components are inherently sensitive to environmental conditions including temperature, humidity, and light exposure. This vulnerability often necessitates additional protective measures that increase complexity and cost. Scalability presents another major hurdle, as many current bioelectronic solutions require specialized manufacturing processes that are difficult to implement at industrial scales.
Standardization across the industry is notably lacking, with different proprietary systems using incompatible verification methods. This fragmentation impedes widespread adoption and increases implementation costs. Detection reliability also remains problematic, with some systems showing unacceptable false positive or false negative rates in field conditions.
Regulatory frameworks for bioelectronic authentication technologies are still evolving, creating uncertainty for manufacturers and implementers. Concerns regarding potential environmental impacts of bioelectronic tags, particularly those containing genetically modified materials, have also emerged as adoption increases.
Cost considerations represent perhaps the most significant barrier to widespread implementation. Current bioelectronic anti-counterfeiting solutions typically cost substantially more than conventional methods, limiting their application to high-value products where the authentication premium can be justified.
Current Bioelectronic Anti-Counterfeiting Solutions
01 Neural-electronic interfaces for biosensing
Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for real-time monitoring of neural activity. The technology incorporates specialized electrodes and transducers that can detect and transmit neural signals with high fidelity, providing valuable data for medical diagnostics and neural research.- Neural-electronic interfaces for biosensing: Bioelectronic interfaces that connect neural tissues with electronic devices for biosensing applications. These interfaces enable direct communication between biological neural systems and electronic circuits, allowing for real-time monitoring of neural activity. The technology incorporates specialized electrodes and transducers that can detect and transmit neural signals with high fidelity, providing valuable data for medical diagnostics and neural research.
- Implantable bioelectronic devices: Implantable bioelectronic interfaces designed to integrate with living tissues for therapeutic or monitoring purposes. These devices feature biocompatible materials and specialized coatings that reduce rejection and promote long-term functionality within the body. The technology includes power management systems for sustained operation and wireless communication capabilities for data transmission without invasive procedures.
- Molecular bioelectronic interfaces: Interfaces that operate at the molecular level, connecting biological molecules with electronic components. These interfaces utilize specialized biomolecules like proteins or DNA as functional components in electronic circuits. The technology enables highly specific detection of biological targets through molecular recognition events that generate measurable electronic signals, with applications in diagnostics and environmental monitoring.
- Flexible and wearable bioelectronic interfaces: Bioelectronic interfaces designed with flexibility and wearability for non-invasive biological monitoring. These interfaces incorporate stretchable electronics and conductive polymers that conform to biological surfaces while maintaining electronic functionality. The technology enables continuous monitoring of physiological parameters through skin contact or minimally invasive methods, suitable for healthcare monitoring and fitness applications.
- Bioelectronic interfaces for drug delivery: Specialized bioelectronic interfaces that combine sensing capabilities with controlled drug delivery mechanisms. These systems monitor biological parameters and respond by releasing therapeutic agents at precise dosages and timing. The technology incorporates microfluidic components, stimuli-responsive materials, and electronic control systems to enable closed-loop drug delivery based on real-time physiological feedback.
02 Implantable bioelectronic devices
Implantable bioelectronic interfaces designed for long-term integration with biological tissues. These devices are engineered with biocompatible materials and specialized coatings to minimize immune responses and enhance integration with surrounding tissues. The technology includes power management systems, wireless communication capabilities, and miniaturized components that enable continuous operation within the body for therapeutic and monitoring purposes.Expand Specific Solutions03 Flexible and stretchable bioelectronic materials
Advanced materials engineered for flexibility and stretchability in bioelectronic interfaces. These materials can conform to the irregular surfaces of biological tissues while maintaining electronic functionality. The technology incorporates conductive polymers, nanomaterials, and specialized fabrication techniques to create interfaces that can withstand mechanical deformation while providing stable electrical connections with biological systems.Expand Specific Solutions04 Molecular-level bioelectronic interfaces
Bioelectronic interfaces that operate at the molecular level, enabling direct interaction with cellular components. These interfaces utilize biomolecules such as proteins, DNA, and enzymes as functional elements to bridge the gap between electronic devices and biological systems. The technology allows for highly specific detection of biological molecules and can translate biological signals into electronic outputs with minimal signal loss.Expand Specific Solutions05 Wireless bioelectronic communication systems
Wireless communication systems specifically designed for bioelectronic interfaces. These systems enable data transmission between implanted bioelectronic devices and external receivers without physical connections. The technology incorporates low-power radio frequency transmitters, near-field communication protocols, and specialized antennas optimized for biological environments, allowing for real-time monitoring and control of bioelectronic interfaces without compromising tissue integrity.Expand Specific Solutions
Key Industry Players in Bioelectronic Authentication
Bioelectronic interfaces are emerging as a critical component in anti-counterfeiting technologies, currently in the growth phase with an expanding market estimated to reach several billion dollars by 2025. The technology maturity varies across applications, with companies demonstrating different levels of advancement. Industry leaders like Huawei, IBM, and Apple are developing sophisticated solutions integrating bioelectronic interfaces with digital authentication systems, while specialized entities such as Zortag and Feitian Technologies focus on innovative biomarker-based verification methods. Research institutions including the Institute of Automation Chinese Academy of Sciences and Cornell University are pioneering next-generation bioelectronic authentication technologies, pushing the boundaries of what's possible in product security and verification.
International Business Machines Corp.
Technical Solution: IBM has pioneered bioelectronic anti-counterfeiting technology through their "crypto-anchor" system that merges biological markers with blockchain verification. Their approach uses microscopic biological tags containing DNA-like patterns that can be embedded into products and packaging. These biomarkers interact with specialized electronic readers that capture unique biological signatures and convert them to digital authentication codes. The system integrates with IBM's blockchain platform to provide immutable verification records. IBM's technology also incorporates machine learning algorithms that continuously improve detection accuracy by analyzing patterns in authentication attempts. The bioelectronic interface allows for non-invasive verification while maintaining the integrity of the biological components, creating a system that combines the unpredictability of biological variations with the security of digital cryptography.
Strengths: Integration with blockchain provides additional security and traceability; scalable enterprise-level solution with robust backend infrastructure; continuous improvement through machine learning. Weaknesses: Requires investment in specialized reading equipment; implementation complexity may be challenging for smaller organizations.
Shanghai Institute of Applied Physics, Chinese Academy of Sciences
Technical Solution: The Shanghai Institute of Applied Physics has developed a groundbreaking bioelectronic anti-counterfeiting technology that utilizes protein-based molecular switches integrated with nanoscale electronic circuits. Their approach embeds bioelectronic interfaces directly into packaging or products, where specific biological molecules interact with electronic components to generate unique authentication signatures. The system works by utilizing the conformational changes of proteins when exposed to specific triggers, which alters their electrical conductivity in predictable but complex patterns. These patterns are then read by specialized electronic sensors and compared against secure databases for verification. The institute has also pioneered self-powered bioelectronic tags that harvest energy from environmental factors like temperature differentials or mechanical movement, eliminating the need for batteries or external power sources. This creates a sustainable, long-lasting authentication mechanism that combines the complexity of biological systems with the reliability of electronic verification.
Strengths: Self-powered capability provides extended operational lifetime; extremely difficult to counterfeit due to the complex biological-electronic interactions; scalable from individual item authentication to batch verification. Weaknesses: May require specialized expertise for implementation and maintenance; potential challenges in standardization across different manufacturing environments.
Core Patents and Technical Literature Analysis
A method of detection of unauthorized intervention in an identification structure which is designed to aid such detection
PatentPendingUS20240338545A1
Innovation
- A multi-layered identification structure incorporating optical, physical, and electrical elements that interact with interventions to cause detectable alterations, allowing for real-time monitoring and unified branding, including security features for loss prevention and item-level tracking.
A colloidal quantum dots (QDS) based ion sensitive field effect transistor
PatentActiveIN201911049752A
Innovation
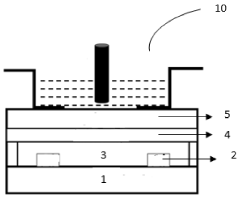
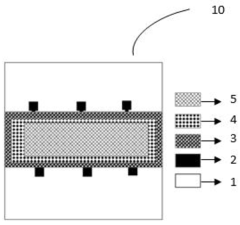
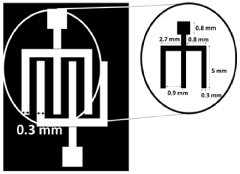
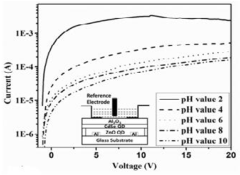
- A colloidal ZnO quantum dots (QDs) based ion sensitive field effect transistor (ISFET) with an interdigitated electrode and a CdSe QDs capping layer, which enhances stability and charge transfer, is developed using a method involving thermal evaporation and colloidal nanosynthesis, where the CdSe QDs protect the ZnO QDs from hydrogenation and improve the device's response to pH variations.
Security and Privacy Implications of Bioelectronic Interfaces
The integration of bioelectronic interfaces in anti-counterfeiting technologies introduces significant security and privacy concerns that must be carefully addressed. As these interfaces collect and process biological data, they create new vulnerabilities that malicious actors could potentially exploit. One primary concern is the protection of biometric data collected through these interfaces, which may include fingerprints, retinal scans, or even neural signals. Unlike passwords or tokens, biometric identifiers cannot be changed if compromised, making their protection paramount.
Data transmission between bioelectronic interfaces and verification systems presents another critical security challenge. Without proper encryption and secure communication protocols, intercepted biological data could be used for unauthorized access or identity theft. The potential for "replay attacks," where previously captured biometric signals are reused to bypass authentication systems, represents a particularly concerning vulnerability unique to bioelectronic anti-counterfeiting measures.
Privacy implications extend beyond data security to questions of consent and transparency. Users may not fully understand what biological information is being collected, how it's being stored, or who has access to it. This raises ethical concerns about informed consent, especially when bioelectronic interfaces become embedded in everyday consumer products or identification systems. The potential for function creep—where collected data is later used for purposes beyond anti-counterfeiting—presents additional privacy risks.
Regulatory frameworks for bioelectronic data protection remain underdeveloped in many jurisdictions, creating uncertainty about legal protections. The cross-border nature of supply chains further complicates compliance with varying privacy regulations, potentially creating security gaps where bioelectronic anti-counterfeiting systems operate across multiple regulatory environments.
The permanence of biological identifiers also raises long-term privacy concerns. Unlike digital credentials that can be revoked or updated, compromised biometric data represents a persistent vulnerability. This necessitates the development of revocable biometric systems and template protection schemes specifically designed for bioelectronic interfaces in anti-counterfeiting applications.
Addressing these security and privacy challenges requires a multi-layered approach combining technical safeguards, clear governance frameworks, and user education. Emerging techniques such as homomorphic encryption, which allows computation on encrypted data, and zero-knowledge proofs that verify authenticity without revealing underlying data, show promise for enhancing privacy while maintaining the effectiveness of bioelectronic anti-counterfeiting measures.
Data transmission between bioelectronic interfaces and verification systems presents another critical security challenge. Without proper encryption and secure communication protocols, intercepted biological data could be used for unauthorized access or identity theft. The potential for "replay attacks," where previously captured biometric signals are reused to bypass authentication systems, represents a particularly concerning vulnerability unique to bioelectronic anti-counterfeiting measures.
Privacy implications extend beyond data security to questions of consent and transparency. Users may not fully understand what biological information is being collected, how it's being stored, or who has access to it. This raises ethical concerns about informed consent, especially when bioelectronic interfaces become embedded in everyday consumer products or identification systems. The potential for function creep—where collected data is later used for purposes beyond anti-counterfeiting—presents additional privacy risks.
Regulatory frameworks for bioelectronic data protection remain underdeveloped in many jurisdictions, creating uncertainty about legal protections. The cross-border nature of supply chains further complicates compliance with varying privacy regulations, potentially creating security gaps where bioelectronic anti-counterfeiting systems operate across multiple regulatory environments.
The permanence of biological identifiers also raises long-term privacy concerns. Unlike digital credentials that can be revoked or updated, compromised biometric data represents a persistent vulnerability. This necessitates the development of revocable biometric systems and template protection schemes specifically designed for bioelectronic interfaces in anti-counterfeiting applications.
Addressing these security and privacy challenges requires a multi-layered approach combining technical safeguards, clear governance frameworks, and user education. Emerging techniques such as homomorphic encryption, which allows computation on encrypted data, and zero-knowledge proofs that verify authenticity without revealing underlying data, show promise for enhancing privacy while maintaining the effectiveness of bioelectronic anti-counterfeiting measures.
Standardization and Interoperability Considerations
The integration of bioelectronic interfaces into anti-counterfeiting technologies necessitates robust standardization and interoperability frameworks to ensure widespread adoption and effectiveness. Currently, the field lacks comprehensive standards specifically addressing bioelectronic authentication methods, creating significant barriers to implementation across global supply chains and verification systems.
Industry stakeholders, including the International Organization for Standardization (ISO) and the Institute of Electrical and Electronics Engineers (IEEE), have begun developing preliminary guidelines for bioelectronic sensing technologies. These efforts focus on establishing uniform protocols for data acquisition, signal processing, and authentication parameters when biological materials interact with electronic detection systems.
Interoperability remains a critical challenge as bioelectronic anti-counterfeiting solutions must function seamlessly across diverse technological ecosystems. The ability of these systems to communicate with existing product authentication databases, blockchain networks, and supply chain management software determines their practical utility. Current implementations often operate as proprietary systems, limiting their integration potential and creating verification silos.
Data format standardization represents another crucial consideration. The unique signatures generated through bioelectronic interfaces—whether derived from protein interactions, DNA hybridization events, or cellular responses—require consistent encoding and transmission protocols. Efforts to establish standardized data structures for bioelectronic authentication signals would significantly enhance cross-platform verification capabilities.
Security standards for bioelectronic interfaces present unique challenges compared to conventional electronic anti-counterfeiting methods. The biological components introduce additional variables requiring specialized protocols for ensuring data integrity, preventing spoofing attacks, and maintaining authentication reliability over product lifecycles. Organizations like the Bioelectronics Standards Advisory Group have proposed initial security frameworks specifically addressing these hybrid biological-electronic systems.
Regulatory considerations further complicate standardization efforts. Bioelectronic interfaces often incorporate materials subject to varying regulations across jurisdictions, particularly when utilizing synthetic biology components or genetically engineered elements. Harmonizing these regulatory requirements with technical standards represents an ongoing challenge for the industry.
The development of open reference architectures would accelerate adoption by providing implementation blueprints for manufacturers and verification authorities. Such architectures would define standard interfaces between biological recognition elements, electronic transduction components, and digital authentication systems, enabling modular development approaches and technology evolution without disrupting existing verification infrastructures.
Cross-industry collaboration between biotechnology firms, electronics manufacturers, and anti-counterfeiting solution providers is essential for establishing meaningful standards. Recent industry consortia have begun addressing these needs, though comprehensive frameworks remain in early development stages.
Industry stakeholders, including the International Organization for Standardization (ISO) and the Institute of Electrical and Electronics Engineers (IEEE), have begun developing preliminary guidelines for bioelectronic sensing technologies. These efforts focus on establishing uniform protocols for data acquisition, signal processing, and authentication parameters when biological materials interact with electronic detection systems.
Interoperability remains a critical challenge as bioelectronic anti-counterfeiting solutions must function seamlessly across diverse technological ecosystems. The ability of these systems to communicate with existing product authentication databases, blockchain networks, and supply chain management software determines their practical utility. Current implementations often operate as proprietary systems, limiting their integration potential and creating verification silos.
Data format standardization represents another crucial consideration. The unique signatures generated through bioelectronic interfaces—whether derived from protein interactions, DNA hybridization events, or cellular responses—require consistent encoding and transmission protocols. Efforts to establish standardized data structures for bioelectronic authentication signals would significantly enhance cross-platform verification capabilities.
Security standards for bioelectronic interfaces present unique challenges compared to conventional electronic anti-counterfeiting methods. The biological components introduce additional variables requiring specialized protocols for ensuring data integrity, preventing spoofing attacks, and maintaining authentication reliability over product lifecycles. Organizations like the Bioelectronics Standards Advisory Group have proposed initial security frameworks specifically addressing these hybrid biological-electronic systems.
Regulatory considerations further complicate standardization efforts. Bioelectronic interfaces often incorporate materials subject to varying regulations across jurisdictions, particularly when utilizing synthetic biology components or genetically engineered elements. Harmonizing these regulatory requirements with technical standards represents an ongoing challenge for the industry.
The development of open reference architectures would accelerate adoption by providing implementation blueprints for manufacturers and verification authorities. Such architectures would define standard interfaces between biological recognition elements, electronic transduction components, and digital authentication systems, enabling modular development approaches and technology evolution without disrupting existing verification infrastructures.
Cross-industry collaboration between biotechnology firms, electronics manufacturers, and anti-counterfeiting solution providers is essential for establishing meaningful standards. Recent industry consortia have begun addressing these needs, though comprehensive frameworks remain in early development stages.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!