Investigation into Antibacterial Coatings for Surgical Instruments
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antibacterial Coating Technology Background and Objectives
The evolution of antibacterial coatings for surgical instruments has been a critical area of development in medical technology over the past several decades. Initially emerging from basic sterilization techniques in the late 19th century, these technologies have progressed significantly through various scientific breakthroughs in materials science, microbiology, and nanotechnology. The trajectory has moved from simple chemical treatments to sophisticated multi-functional coatings that actively combat bacterial colonization while maintaining instrument functionality.
Recent advancements have been driven by the growing concern over healthcare-associated infections (HAIs), particularly surgical site infections (SSIs) which affect approximately 2-5% of patients undergoing surgical procedures globally. The economic burden of these infections exceeds $3.5 billion annually in the United States alone, creating substantial market pressure for effective preventative technologies.
The technical evolution path has witnessed several paradigm shifts, from passive barrier coatings to active antimicrobial surfaces, and now toward smart responsive systems. Early approaches relied primarily on silver and copper ions for their inherent antimicrobial properties, while contemporary solutions incorporate advanced materials such as quaternary ammonium compounds, chitosan derivatives, and metal oxide nanoparticles that provide enhanced efficacy against a broader spectrum of pathogens.
A significant milestone was reached in the early 2000s with the development of hydrophobic and superhydrophobic surfaces that prevent bacterial adhesion through physical rather than chemical means. This approach addressed growing concerns about antimicrobial resistance while providing longer-lasting protection for instruments subjected to repeated sterilization cycles.
The primary technical objectives in this field now center on developing coatings that demonstrate several critical characteristics: broad-spectrum antimicrobial activity, minimal cytotoxicity to human tissues, mechanical durability under clinical use conditions, compatibility with standard sterilization protocols, and cost-effectiveness for widespread implementation. Additionally, there is increasing focus on creating solutions that remain effective against biofilm formation, which represents a particularly challenging aspect of bacterial contamination.
Looking forward, the field is moving toward multifunctional coatings that combine antimicrobial properties with additional benefits such as anti-fouling capabilities, reduced friction, enhanced visibility under surgical conditions, and even integration with smart technologies for real-time contamination monitoring. The ultimate goal remains the development of sustainable, long-lasting solutions that significantly reduce infection rates while meeting the practical demands of modern surgical environments.
Recent advancements have been driven by the growing concern over healthcare-associated infections (HAIs), particularly surgical site infections (SSIs) which affect approximately 2-5% of patients undergoing surgical procedures globally. The economic burden of these infections exceeds $3.5 billion annually in the United States alone, creating substantial market pressure for effective preventative technologies.
The technical evolution path has witnessed several paradigm shifts, from passive barrier coatings to active antimicrobial surfaces, and now toward smart responsive systems. Early approaches relied primarily on silver and copper ions for their inherent antimicrobial properties, while contemporary solutions incorporate advanced materials such as quaternary ammonium compounds, chitosan derivatives, and metal oxide nanoparticles that provide enhanced efficacy against a broader spectrum of pathogens.
A significant milestone was reached in the early 2000s with the development of hydrophobic and superhydrophobic surfaces that prevent bacterial adhesion through physical rather than chemical means. This approach addressed growing concerns about antimicrobial resistance while providing longer-lasting protection for instruments subjected to repeated sterilization cycles.
The primary technical objectives in this field now center on developing coatings that demonstrate several critical characteristics: broad-spectrum antimicrobial activity, minimal cytotoxicity to human tissues, mechanical durability under clinical use conditions, compatibility with standard sterilization protocols, and cost-effectiveness for widespread implementation. Additionally, there is increasing focus on creating solutions that remain effective against biofilm formation, which represents a particularly challenging aspect of bacterial contamination.
Looking forward, the field is moving toward multifunctional coatings that combine antimicrobial properties with additional benefits such as anti-fouling capabilities, reduced friction, enhanced visibility under surgical conditions, and even integration with smart technologies for real-time contamination monitoring. The ultimate goal remains the development of sustainable, long-lasting solutions that significantly reduce infection rates while meeting the practical demands of modern surgical environments.
Market Analysis for Antimicrobial Surgical Instruments
The global market for antimicrobial surgical instruments has been experiencing significant growth, driven primarily by increasing concerns over healthcare-associated infections (HAIs) and surgical site infections (SSIs). Current market valuation stands at approximately 4.2 billion USD as of 2023, with projections indicating a compound annual growth rate (CAGR) of 7.3% through 2028. This growth trajectory is supported by heightened awareness of infection control protocols in healthcare settings worldwide.
North America currently dominates the market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. The remaining regions collectively account for 9% of the global market. Within these regions, hospitals represent the largest end-user segment (62%), followed by ambulatory surgical centers (21%) and specialty clinics (17%).
Demand patterns reveal a strong preference for antimicrobial-coated stainless steel instruments, which constitute approximately 45% of the market. Titanium-based instruments with antimicrobial properties account for 28%, while polymer-based instruments with embedded antimicrobial agents represent 18%. The remaining 9% includes specialized materials and experimental coatings currently undergoing clinical validation.
Key market drivers include the rising incidence of surgical procedures globally, with over 310 million major surgeries performed annually. Additionally, the economic burden of SSIs, estimated at 3.3 billion USD annually in the United States alone, has prompted healthcare facilities to invest in preventative technologies. Regulatory bodies have also intensified their focus on infection control measures, creating favorable market conditions for antimicrobial surgical instruments.
Consumer behavior analysis indicates that purchasing decisions are primarily influenced by clinical efficacy data (42%), cost-effectiveness (27%), durability of antimicrobial properties (18%), and compatibility with existing sterilization protocols (13%). Healthcare facilities increasingly demand solutions that offer long-lasting antimicrobial protection without compromising instrument functionality or requiring significant changes to established workflows.
Market segmentation by antimicrobial coating type shows silver-based coatings leading with 37% market share, followed by copper-based (22%), zinc-based (15%), and polymer-based antimicrobial systems (14%). Emerging technologies, including photoactive antimicrobial coatings and enzyme-based systems, collectively represent 12% of the market but are experiencing the fastest growth rates.
Pricing trends indicate a premium of 15-30% for antimicrobial instruments compared to standard counterparts, though this gap is narrowing as manufacturing processes become more efficient and competition intensifies. The market is expected to reach price equilibrium within the next 3-5 years as technology matures and production scales.
North America currently dominates the market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. The remaining regions collectively account for 9% of the global market. Within these regions, hospitals represent the largest end-user segment (62%), followed by ambulatory surgical centers (21%) and specialty clinics (17%).
Demand patterns reveal a strong preference for antimicrobial-coated stainless steel instruments, which constitute approximately 45% of the market. Titanium-based instruments with antimicrobial properties account for 28%, while polymer-based instruments with embedded antimicrobial agents represent 18%. The remaining 9% includes specialized materials and experimental coatings currently undergoing clinical validation.
Key market drivers include the rising incidence of surgical procedures globally, with over 310 million major surgeries performed annually. Additionally, the economic burden of SSIs, estimated at 3.3 billion USD annually in the United States alone, has prompted healthcare facilities to invest in preventative technologies. Regulatory bodies have also intensified their focus on infection control measures, creating favorable market conditions for antimicrobial surgical instruments.
Consumer behavior analysis indicates that purchasing decisions are primarily influenced by clinical efficacy data (42%), cost-effectiveness (27%), durability of antimicrobial properties (18%), and compatibility with existing sterilization protocols (13%). Healthcare facilities increasingly demand solutions that offer long-lasting antimicrobial protection without compromising instrument functionality or requiring significant changes to established workflows.
Market segmentation by antimicrobial coating type shows silver-based coatings leading with 37% market share, followed by copper-based (22%), zinc-based (15%), and polymer-based antimicrobial systems (14%). Emerging technologies, including photoactive antimicrobial coatings and enzyme-based systems, collectively represent 12% of the market but are experiencing the fastest growth rates.
Pricing trends indicate a premium of 15-30% for antimicrobial instruments compared to standard counterparts, though this gap is narrowing as manufacturing processes become more efficient and competition intensifies. The market is expected to reach price equilibrium within the next 3-5 years as technology matures and production scales.
Current Antibacterial Coating Technologies and Challenges
The global market for antibacterial coatings on surgical instruments is currently dominated by several key technologies, each with distinct advantages and limitations. Silver-based coatings represent the most widely adopted solution, leveraging silver's well-documented antimicrobial properties through various delivery mechanisms including silver nanoparticles, silver ions, and silver-polymer composites. These coatings have demonstrated efficacy against a broad spectrum of pathogens, including antibiotic-resistant strains, with relatively low toxicity profiles.
Copper and copper alloy coatings have emerged as cost-effective alternatives, exhibiting strong antimicrobial activity through contact killing mechanisms. Research indicates copper ions disrupt bacterial cell membranes and denature proteins, providing continuous protection without significant degradation over time. However, these coatings face challenges related to durability during repeated sterilization cycles and potential discoloration issues.
Quaternary ammonium compound (QAC) based coatings represent another significant category, functioning through electrostatic interactions that disrupt bacterial cell membranes. These coatings offer advantages in terms of chemical stability and ease of application, though concerns persist regarding their long-term efficacy and potential for contributing to antimicrobial resistance.
Titanium dioxide photocatalytic coatings have gained attention for their self-cleaning properties, activated by light exposure to generate reactive oxygen species that destroy microorganisms. While promising in laboratory settings, their practical application remains limited by the requirement for UV light activation and relatively slow killing kinetics compared to other technologies.
Despite these advances, significant challenges persist across all current antibacterial coating technologies. Durability represents a primary concern, as most coatings demonstrate reduced efficacy after multiple sterilization cycles using autoclave, ethylene oxide, or gamma radiation methods. This degradation compromises long-term protection and increases healthcare costs through more frequent instrument replacement.
Biocompatibility issues present another critical challenge, with some coatings showing potential cytotoxicity or inflammatory responses in surrounding tissues. This is particularly problematic for surgical instruments that come into direct contact with open wounds or internal organs.
Regulatory hurdles further complicate advancement in this field, with stringent approval processes for novel coating technologies varying significantly across global markets. The FDA and European Medical Agency maintain rigorous standards requiring extensive efficacy and safety data, creating substantial barriers to market entry for innovative solutions.
Additionally, the emergence of resistant microbial strains poses an evolving challenge, as some bacteria have demonstrated adaptation to certain antimicrobial agents, particularly those with single-mechanism action. This necessitates the development of multi-modal coating approaches that can overcome adaptive resistance mechanisms.
Copper and copper alloy coatings have emerged as cost-effective alternatives, exhibiting strong antimicrobial activity through contact killing mechanisms. Research indicates copper ions disrupt bacterial cell membranes and denature proteins, providing continuous protection without significant degradation over time. However, these coatings face challenges related to durability during repeated sterilization cycles and potential discoloration issues.
Quaternary ammonium compound (QAC) based coatings represent another significant category, functioning through electrostatic interactions that disrupt bacterial cell membranes. These coatings offer advantages in terms of chemical stability and ease of application, though concerns persist regarding their long-term efficacy and potential for contributing to antimicrobial resistance.
Titanium dioxide photocatalytic coatings have gained attention for their self-cleaning properties, activated by light exposure to generate reactive oxygen species that destroy microorganisms. While promising in laboratory settings, their practical application remains limited by the requirement for UV light activation and relatively slow killing kinetics compared to other technologies.
Despite these advances, significant challenges persist across all current antibacterial coating technologies. Durability represents a primary concern, as most coatings demonstrate reduced efficacy after multiple sterilization cycles using autoclave, ethylene oxide, or gamma radiation methods. This degradation compromises long-term protection and increases healthcare costs through more frequent instrument replacement.
Biocompatibility issues present another critical challenge, with some coatings showing potential cytotoxicity or inflammatory responses in surrounding tissues. This is particularly problematic for surgical instruments that come into direct contact with open wounds or internal organs.
Regulatory hurdles further complicate advancement in this field, with stringent approval processes for novel coating technologies varying significantly across global markets. The FDA and European Medical Agency maintain rigorous standards requiring extensive efficacy and safety data, creating substantial barriers to market entry for innovative solutions.
Additionally, the emergence of resistant microbial strains poses an evolving challenge, as some bacteria have demonstrated adaptation to certain antimicrobial agents, particularly those with single-mechanism action. This necessitates the development of multi-modal coating approaches that can overcome adaptive resistance mechanisms.
Current Antibacterial Coating Solutions for Surgical Tools
01 Metal-based antibacterial coatings
Metal-based antibacterial coatings utilize silver, copper, zinc, or other metal ions to inhibit bacterial growth on surfaces. These metals release ions that disrupt bacterial cell membranes and interfere with cellular processes. Such coatings can be applied to medical devices, household items, and industrial equipment to provide long-lasting antimicrobial protection. The metals can be incorporated as nanoparticles, oxides, or complexes within various coating matrices to enhance their efficacy and durability.- Metal-based antibacterial coatings: Metal-based antibacterial coatings utilize metals such as silver, copper, and zinc that have inherent antimicrobial properties. These metals can be incorporated into coatings as nanoparticles, ions, or compounds that slowly release active metal ions to inhibit bacterial growth. The mechanism typically involves disruption of bacterial cell membranes, interference with enzyme functions, or generation of reactive oxygen species. These coatings are particularly effective for medical devices, food processing equipment, and high-touch surfaces in healthcare settings.
- Polymer-based antibacterial coatings: Polymer-based antibacterial coatings incorporate antimicrobial agents within polymer matrices to create surfaces that resist bacterial colonization. These polymers can be designed to either release antimicrobial compounds gradually or to have inherent antibacterial properties through positively charged functional groups that disrupt bacterial cell membranes. Common polymers used include quaternary ammonium-containing polymers, chitosan derivatives, and polyethylene glycol. These coatings provide durable protection and can be applied to various substrates including textiles, plastics, and metals.
- Natural compound-based antibacterial coatings: Natural compound-based antibacterial coatings utilize plant extracts, essential oils, enzymes, and other naturally derived substances with antimicrobial properties. These environmentally friendly alternatives often contain compounds like polyphenols, terpenes, and flavonoids that can inhibit bacterial growth through multiple mechanisms. The coatings can be formulated as films, sprays, or incorporated into matrices for controlled release. They are particularly valuable in food packaging, consumer products, and applications where toxicity concerns or environmental impact are important considerations.
- Photocatalytic antibacterial coatings: Photocatalytic antibacterial coatings contain materials such as titanium dioxide (TiO2) or zinc oxide that generate reactive oxygen species when exposed to light. These reactive species can effectively kill bacteria by oxidizing their cell components. The coatings remain active as long as they receive appropriate light exposure, providing continuous disinfection without depleting the active ingredients. These self-cleaning surfaces are particularly useful for environmental applications, building materials, and public spaces where regular exposure to light can be expected.
- Smart responsive antibacterial coatings: Smart responsive antibacterial coatings are designed to activate their antimicrobial properties in response to specific triggers such as changes in pH, temperature, bacterial presence, or external stimuli. These advanced coatings can remain dormant until needed, which helps prevent antimicrobial resistance and extends their effective lifespan. Some designs incorporate bacteria-responsive elements that release antimicrobial agents only when pathogens are detected. This targeted approach minimizes unnecessary exposure to antimicrobial agents and provides more efficient protection in medical devices, implants, and wound dressings.
02 Polymer-based antibacterial coatings
Polymer-based antibacterial coatings incorporate antimicrobial agents within polymer matrices to create protective surfaces. These polymers can be designed to slowly release active ingredients or to have inherent antibacterial properties through positively charged functional groups that disrupt bacterial cell membranes. Common polymers used include quaternary ammonium compounds, chitosan derivatives, and synthetic polymers with antimicrobial properties. These coatings provide durable protection for various surfaces including medical devices, textiles, and consumer products.Expand Specific Solutions03 Natural compound-based antibacterial coatings
Antibacterial coatings derived from natural compounds utilize plant extracts, essential oils, enzymes, and other biological materials to inhibit bacterial growth. These environmentally friendly alternatives offer reduced toxicity compared to synthetic options. Natural compounds like chitosan, tea tree oil, oregano oil, and various plant polyphenols can be incorporated into coating formulations. These coatings are particularly valuable in food packaging, medical applications, and consumer products where biocompatibility and sustainability are important considerations.Expand Specific Solutions04 Photocatalytic antibacterial coatings
Photocatalytic antibacterial coatings utilize materials that generate reactive oxygen species when exposed to light, which then destroy bacteria on contact. Titanium dioxide is commonly used in these coatings, along with other semiconductor materials that can be activated by visible or UV light. These coatings provide continuous antibacterial action as long as they receive light exposure. Applications include self-cleaning surfaces, water treatment systems, air purification devices, and hospital environments where maintaining sterile conditions is critical.Expand Specific Solutions05 Multi-functional antibacterial coatings
Multi-functional antibacterial coatings combine antimicrobial properties with additional beneficial characteristics such as anti-fouling, self-cleaning, or enhanced durability. These advanced coatings often incorporate multiple active ingredients or utilize sophisticated delivery systems to provide sustained protection. They may combine different antibacterial mechanisms to prevent bacterial resistance or include components that respond to environmental triggers. Applications include medical implants, marine equipment, industrial surfaces, and consumer electronics where multiple surface properties are desired simultaneously.Expand Specific Solutions
Leading Manufacturers and Research Institutions Analysis
The antibacterial coatings for surgical instruments market is in a growth phase, driven by increasing healthcare-associated infections and surgical site infections globally. The market size is expanding rapidly, estimated to reach several billion dollars by 2025 with a CAGR of 10-12%. Regarding technological maturity, established players like Ethicon (Johnson & Johnson), Becton Dickinson, and Medtronic lead with advanced antimicrobial coating technologies, while research institutions such as Baylor College of Medicine, University of South Australia, and Fraunhofer-Gesellschaft are developing next-generation solutions. Emerging companies like Orthobond Corp. and Bio-Gate AG are introducing innovative approaches using nanotechnology and silver-based antimicrobials. Regional players such as Jiangsu Biosurf Biotech and Shandong Weigao Group are strengthening Asia's market position with cost-effective solutions.
Ethicon, Inc.
Technical Solution: Ethicon, Inc., a Johnson & Johnson company, has developed the Plus Antibacterial Suture technology that incorporates Triclosan (Irgacare MP) into the coating of surgical instruments and sutures. This proprietary technology involves a zone of inhibition that prevents bacterial colonization on the instrument surface. The coating process utilizes a specialized polymer matrix that allows for controlled release of the antimicrobial agent while maintaining instrument integrity and functionality. Independent clinical studies have demonstrated that Ethicon's antibacterial coatings reduce surgical site infections by approximately 30% compared to procedures using standard instruments[6]. The technology has been particularly effective against Staphylococcus aureus and Staphylococcus epidermidis, two common causes of surgical site infections. Ethicon has expanded this technology beyond sutures to various surgical instruments, developing specialized application techniques for different instrument materials and geometries. Their latest generation coating incorporates nanotechnology to enhance durability through multiple sterilization cycles while maintaining antimicrobial efficacy. The company has also developed a comprehensive testing protocol to verify coating integrity and antimicrobial activity after repeated clinical use, ensuring consistent performance throughout the instrument's lifecycle[7].
Strengths: Extensive clinical validation with documented reduction in surgical site infections; established regulatory pathway with multiple approvals; seamless integration into existing surgical workflows; minimal impact on instrument handling characteristics. Weaknesses: Growing concerns about triclosan environmental impact and potential for antimicrobial resistance; gradual reduction in efficacy after multiple sterilization cycles; limited spectrum of activity against gram-negative bacteria and fungi; potential for coating damage during aggressive cleaning procedures.
BASF Corp.
Technical Solution: BASF Corporation has developed an innovative antimicrobial coating system for surgical instruments under their HyGentic™ product line. This technology utilizes a combination of quaternary ammonium compounds and copper nanoparticles embedded in a specialized polymer matrix. The dual-action mechanism provides both immediate contact killing and sustained antimicrobial activity through the controlled release of copper ions. BASF's proprietary application process involves a base layer that promotes adhesion to metal surfaces, followed by the active antimicrobial layer, and finally a top coat that controls release kinetics while enhancing durability. Laboratory testing has demonstrated efficacy against a wide range of pathogens including antibiotic-resistant strains such as MRSA and VRE, with bacterial reduction rates exceeding 99.99% within 2 hours of exposure[5]. The coating maintains its antimicrobial properties through at least 50 sterilization cycles using standard hospital protocols. BASF has also developed specialized formulations for different surgical specialties, with cardiovascular and neurological instruments receiving tailored coatings that address the specific microbial challenges in these fields. The company's research has shown that instruments treated with HyGentic™ coatings significantly reduce biofilm formation in simulated clinical environments, potentially addressing one of the most challenging aspects of instrument-related infections.
Strengths: Dual-mechanism antimicrobial action provides both immediate and sustained protection; excellent adhesion to various metal alloys used in surgical instruments; demonstrated efficacy against biofilm formation; compatible with multiple sterilization methods. Weaknesses: Gradual reduction in antimicrobial efficacy after multiple sterilization cycles; potential for copper ion accumulation in tissues with repeated use; more complex regulatory pathway due to novel material combinations; higher production costs compared to conventional instruments.
Key Patents and Research in Antimicrobial Surface Technology
Antimicrobial coatings
PatentWO2020035483A1
Innovation
- An antimicrobial liquid crystal composition comprising amphiphilic lipids, antimicrobial agents, and water that forms stable liquid crystals at room temperature, providing sustained and adhesive antimicrobial properties without requiring additional surfactants or polymers, and can be adapted by stimuli like humidity and pH to enhance antimicrobial efficacy.
Antimicrobial coating for inhibition of bacterial adhesion and biofilm formation
PatentInactiveUS20150010715A1
Innovation
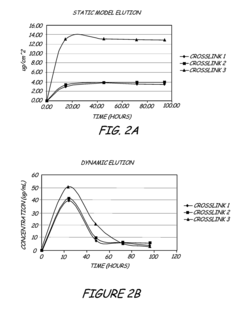
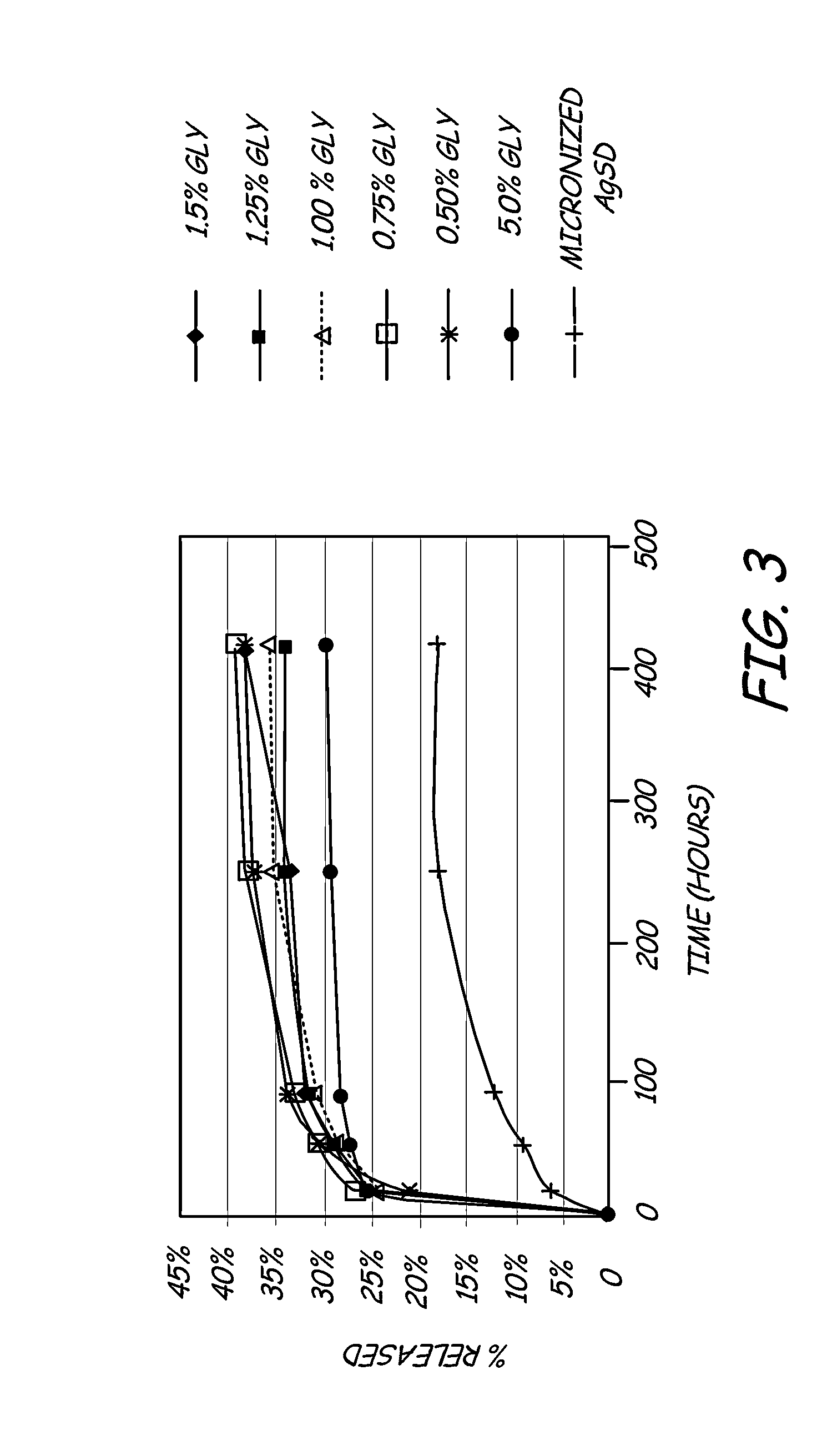
- A hydrophilic polymeric coating with a cross-linked 3D network that maintains a water-insoluble antimicrobial metallic compound, such as silver sulfadiazine, in a solubilized form, allowing high concentrations to be incorporated in thin coatings, inhibiting bacterial adhesion and biofilm formation by releasing bactericidal silver ions.
Biocompatibility and Safety Considerations
The biocompatibility of antibacterial coatings for surgical instruments represents a critical consideration in their development and implementation. These coatings must not only effectively combat bacterial colonization but also demonstrate complete safety when in contact with human tissues during surgical procedures. The primary concern is ensuring that coating materials do not elicit adverse biological responses such as inflammation, allergic reactions, or cytotoxicity when exposed to blood, tissue fluids, or direct tissue contact.
Regulatory frameworks worldwide, including FDA guidelines and ISO 10993 standards, establish rigorous testing protocols for evaluating the biocompatibility of medical device coatings. These assessments typically include cytotoxicity testing, sensitization studies, irritation evaluations, systemic toxicity assessments, and genotoxicity screening. For surgical instruments with antibacterial coatings, additional hemocompatibility testing is essential to ensure the coating does not trigger thrombosis or hemolysis when contacting blood.
Silver-based coatings, while demonstrating excellent antibacterial properties, present specific safety considerations regarding potential silver ion leaching and accumulation in tissues. Research indicates that controlled-release formulations can mitigate these risks while maintaining antimicrobial efficacy. Similarly, copper-based coatings must address concerns about copper toxicity at higher concentrations, necessitating precise control of ion release rates.
Polymer-based antibacterial coatings incorporating quaternary ammonium compounds have shown promising results in laboratory settings, but their long-term biocompatibility remains under investigation. Recent studies suggest that surface-bound antimicrobial agents may offer superior safety profiles compared to leaching systems, as they minimize systemic exposure to antimicrobial agents.
The degradation products of antibacterial coatings represent another critical safety consideration. Coatings must maintain their integrity throughout the intended use period, with any breakdown products being non-toxic and readily eliminated from the body. This is particularly important for biodegradable coating systems, where the degradation timeline must align with the instrument's usage cycle.
Manufacturing processes for antibacterial coatings must also be evaluated for safety implications. Residual solvents, unreacted monomers, or processing additives could potentially remain in the final coating and present toxicity risks. Advanced analytical techniques such as high-performance liquid chromatography and mass spectrometry are employed to detect and quantify such residuals, ensuring they remain below established safety thresholds.
The balance between antimicrobial efficacy and biocompatibility often presents a significant challenge in coating development. Optimizing this balance requires sophisticated formulation strategies and extensive biological testing to ensure that the coating's antibacterial mechanisms do not compromise patient safety or surgical outcomes.
Regulatory frameworks worldwide, including FDA guidelines and ISO 10993 standards, establish rigorous testing protocols for evaluating the biocompatibility of medical device coatings. These assessments typically include cytotoxicity testing, sensitization studies, irritation evaluations, systemic toxicity assessments, and genotoxicity screening. For surgical instruments with antibacterial coatings, additional hemocompatibility testing is essential to ensure the coating does not trigger thrombosis or hemolysis when contacting blood.
Silver-based coatings, while demonstrating excellent antibacterial properties, present specific safety considerations regarding potential silver ion leaching and accumulation in tissues. Research indicates that controlled-release formulations can mitigate these risks while maintaining antimicrobial efficacy. Similarly, copper-based coatings must address concerns about copper toxicity at higher concentrations, necessitating precise control of ion release rates.
Polymer-based antibacterial coatings incorporating quaternary ammonium compounds have shown promising results in laboratory settings, but their long-term biocompatibility remains under investigation. Recent studies suggest that surface-bound antimicrobial agents may offer superior safety profiles compared to leaching systems, as they minimize systemic exposure to antimicrobial agents.
The degradation products of antibacterial coatings represent another critical safety consideration. Coatings must maintain their integrity throughout the intended use period, with any breakdown products being non-toxic and readily eliminated from the body. This is particularly important for biodegradable coating systems, where the degradation timeline must align with the instrument's usage cycle.
Manufacturing processes for antibacterial coatings must also be evaluated for safety implications. Residual solvents, unreacted monomers, or processing additives could potentially remain in the final coating and present toxicity risks. Advanced analytical techniques such as high-performance liquid chromatography and mass spectrometry are employed to detect and quantify such residuals, ensuring they remain below established safety thresholds.
The balance between antimicrobial efficacy and biocompatibility often presents a significant challenge in coating development. Optimizing this balance requires sophisticated formulation strategies and extensive biological testing to ensure that the coating's antibacterial mechanisms do not compromise patient safety or surgical outcomes.
Regulatory Framework for Medical Device Coatings
The regulatory landscape governing antibacterial coatings for surgical instruments is complex and multifaceted, requiring manufacturers to navigate various approval pathways and compliance requirements. In the United States, the Food and Drug Administration (FDA) classifies coated surgical instruments under medical device regulations, with most falling into Class II, requiring a 510(k) premarket notification unless the coating introduces novel antimicrobial agents, which may necessitate a more rigorous Premarket Approval (PMA) process.
The FDA's guidance document "Premarket Notification [510(k)] Submissions for Medical Devices that Include Antimicrobial Agents" specifically addresses requirements for devices with antibacterial properties, emphasizing the need for comprehensive safety and efficacy data. Manufacturers must demonstrate that the coating does not leach harmful substances, maintains effectiveness over the product lifecycle, and does not interfere with the instrument's primary function.
In the European Union, the Medical Device Regulation (MDR 2017/745) has significantly increased requirements for medical devices, including those with antibacterial coatings. Surgical instruments with antimicrobial properties may be classified as Class IIa or higher, depending on their intended use and risk profile. The MDR places particular emphasis on clinical evaluation, post-market surveillance, and the use of unique device identification (UDI) systems.
International Organization for Standardization (ISO) standards play a crucial role in regulatory compliance. ISO 10993 series addresses biocompatibility testing for medical devices, while ISO 22196 specifically evaluates antimicrobial activity on surfaces. These standards provide harmonized testing methodologies that facilitate global market access and regulatory approval.
Regulatory bodies increasingly require manufacturers to conduct leachability and extractable studies to ensure coating stability under various conditions. Environmental considerations are also gaining prominence, with regulations in certain jurisdictions limiting the use of specific antimicrobial agents due to potential ecological impacts or antimicrobial resistance concerns.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory frameworks for antimicrobial medical devices, often requiring country-specific testing and documentation. This regulatory diversity creates significant challenges for global manufacturers seeking multinational approvals.
Recent regulatory trends indicate a move toward more stringent requirements for demonstrating clinical benefit and cost-effectiveness of antimicrobial coatings, particularly in healthcare systems employing health technology assessment (HTA) methodologies. Manufacturers must increasingly provide evidence that their coated instruments contribute meaningfully to infection reduction in clinical settings, not merely demonstrate antimicrobial activity in laboratory conditions.
The FDA's guidance document "Premarket Notification [510(k)] Submissions for Medical Devices that Include Antimicrobial Agents" specifically addresses requirements for devices with antibacterial properties, emphasizing the need for comprehensive safety and efficacy data. Manufacturers must demonstrate that the coating does not leach harmful substances, maintains effectiveness over the product lifecycle, and does not interfere with the instrument's primary function.
In the European Union, the Medical Device Regulation (MDR 2017/745) has significantly increased requirements for medical devices, including those with antibacterial coatings. Surgical instruments with antimicrobial properties may be classified as Class IIa or higher, depending on their intended use and risk profile. The MDR places particular emphasis on clinical evaluation, post-market surveillance, and the use of unique device identification (UDI) systems.
International Organization for Standardization (ISO) standards play a crucial role in regulatory compliance. ISO 10993 series addresses biocompatibility testing for medical devices, while ISO 22196 specifically evaluates antimicrobial activity on surfaces. These standards provide harmonized testing methodologies that facilitate global market access and regulatory approval.
Regulatory bodies increasingly require manufacturers to conduct leachability and extractable studies to ensure coating stability under various conditions. Environmental considerations are also gaining prominence, with regulations in certain jurisdictions limiting the use of specific antimicrobial agents due to potential ecological impacts or antimicrobial resistance concerns.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory frameworks for antimicrobial medical devices, often requiring country-specific testing and documentation. This regulatory diversity creates significant challenges for global manufacturers seeking multinational approvals.
Recent regulatory trends indicate a move toward more stringent requirements for demonstrating clinical benefit and cost-effectiveness of antimicrobial coatings, particularly in healthcare systems employing health technology assessment (HTA) methodologies. Manufacturers must increasingly provide evidence that their coated instruments contribute meaningfully to infection reduction in clinical settings, not merely demonstrate antimicrobial activity in laboratory conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!