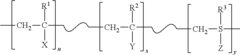
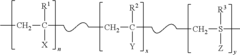
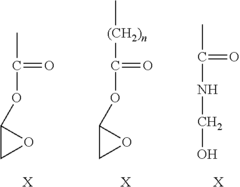
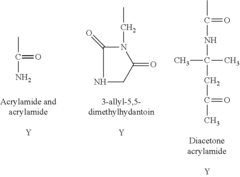
Regulations Governing Antibacterial Coatings in Healthcare
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antibacterial Coating Regulations Background and Objectives
Antibacterial coatings in healthcare settings have emerged as a critical technology in the battle against healthcare-associated infections (HAIs). The evolution of these coatings can be traced back to the early 1990s when initial research focused on silver-based antimicrobial surfaces. Over the subsequent decades, technological advancements have expanded the range of materials and application methods, leading to more sophisticated and effective coating solutions.
The regulatory landscape governing antibacterial coatings has developed in parallel with technological progress. In the United States, the Environmental Protection Agency (EPA) regulates antimicrobial products under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), while the Food and Drug Administration (FDA) oversees medical devices with antimicrobial properties. In Europe, the Biocidal Products Regulation (BPR) and Medical Device Regulation (MDR) provide the regulatory framework for these technologies.
Recent global health crises, particularly the COVID-19 pandemic, have accelerated interest in and development of antibacterial surface technologies. This has prompted regulatory bodies worldwide to reassess and update their frameworks to address emerging technologies while ensuring patient safety remains paramount. The increasing prevalence of antibiotic-resistant bacteria has further emphasized the importance of effective regulation in this domain.
The technical objectives for antibacterial coating regulations center on establishing standardized testing protocols to verify efficacy claims, ensuring long-term durability of antimicrobial properties, and confirming the absence of harmful effects on human health and the environment. Additionally, regulations aim to address the potential development of bacterial resistance to antimicrobial agents used in coatings.
Current regulatory trends indicate a move toward more harmonized international standards, with increasing collaboration between major regulatory bodies such as the FDA, EPA, European Medicines Agency, and various ISO technical committees. This harmonization seeks to reduce regulatory barriers while maintaining rigorous safety and efficacy standards.
The future trajectory of antibacterial coating regulations is likely to incorporate more sophisticated risk-benefit analyses, acknowledging both the potential benefits of reducing infection rates and the possible risks of antimicrobial resistance development. Regulations are expected to evolve toward a more holistic approach that considers the entire lifecycle of coated products, from manufacturing processes to disposal considerations.
As nanotechnology and novel antimicrobial compounds continue to advance, regulatory frameworks must remain adaptable to address these innovations while providing clear guidance to manufacturers and healthcare facilities on implementation requirements and compliance pathways.
The regulatory landscape governing antibacterial coatings has developed in parallel with technological progress. In the United States, the Environmental Protection Agency (EPA) regulates antimicrobial products under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), while the Food and Drug Administration (FDA) oversees medical devices with antimicrobial properties. In Europe, the Biocidal Products Regulation (BPR) and Medical Device Regulation (MDR) provide the regulatory framework for these technologies.
Recent global health crises, particularly the COVID-19 pandemic, have accelerated interest in and development of antibacterial surface technologies. This has prompted regulatory bodies worldwide to reassess and update their frameworks to address emerging technologies while ensuring patient safety remains paramount. The increasing prevalence of antibiotic-resistant bacteria has further emphasized the importance of effective regulation in this domain.
The technical objectives for antibacterial coating regulations center on establishing standardized testing protocols to verify efficacy claims, ensuring long-term durability of antimicrobial properties, and confirming the absence of harmful effects on human health and the environment. Additionally, regulations aim to address the potential development of bacterial resistance to antimicrobial agents used in coatings.
Current regulatory trends indicate a move toward more harmonized international standards, with increasing collaboration between major regulatory bodies such as the FDA, EPA, European Medicines Agency, and various ISO technical committees. This harmonization seeks to reduce regulatory barriers while maintaining rigorous safety and efficacy standards.
The future trajectory of antibacterial coating regulations is likely to incorporate more sophisticated risk-benefit analyses, acknowledging both the potential benefits of reducing infection rates and the possible risks of antimicrobial resistance development. Regulations are expected to evolve toward a more holistic approach that considers the entire lifecycle of coated products, from manufacturing processes to disposal considerations.
As nanotechnology and novel antimicrobial compounds continue to advance, regulatory frameworks must remain adaptable to address these innovations while providing clear guidance to manufacturers and healthcare facilities on implementation requirements and compliance pathways.
Healthcare Market Demand for Antimicrobial Solutions
The healthcare sector has witnessed a significant surge in demand for antimicrobial solutions, particularly in the wake of global health crises and the persistent challenge of healthcare-associated infections (HAIs). Market research indicates that HAIs affect approximately 1.7 million patients annually in the United States alone, resulting in nearly 99,000 deaths and adding an estimated $20 billion in healthcare costs. This substantial burden has created a robust market for antimicrobial technologies, with particular emphasis on antibacterial coatings for healthcare environments.
Hospital administrators and healthcare providers are increasingly recognizing the value proposition of antimicrobial surfaces in reducing pathogen transmission. A recent survey of healthcare facility managers revealed that 78% consider antimicrobial properties as "very important" or "essential" when selecting surface materials for new construction or renovation projects. This represents a marked increase from 52% five years ago, demonstrating the growing awareness of infection control benefits.
The market demand is further driven by evolving patient expectations and healthcare consumer awareness. Modern healthcare consumers are increasingly informed about infection risks and actively seek facilities that demonstrate commitment to advanced infection prevention measures. Healthcare systems that implement comprehensive antimicrobial strategies often report this as a competitive advantage in patient acquisition and retention.
Regulatory pressures also significantly influence market demand. Healthcare facilities face stringent reporting requirements for infection rates, with financial penalties for excessive HAIs under value-based purchasing models. These regulatory frameworks have transformed antimicrobial solutions from optional enhancements to essential risk management tools for healthcare providers seeking to avoid financial penalties and reputation damage.
Regional market analysis reveals varying adoption rates, with North America leading global demand at 38% market share, followed by Europe at 29% and Asia-Pacific showing the fastest growth rate at 14.5% annually. Within these markets, hospitals represent the largest segment (42%), followed by outpatient facilities (27%) and long-term care settings (18%).
The COVID-19 pandemic has accelerated this market trajectory, with 67% of healthcare facilities reporting increased budgets specifically allocated to surface infection control measures. This has expanded beyond traditional high-risk areas like operating rooms and ICUs to encompass all patient care environments, administrative spaces, and public areas within healthcare facilities.
Economic analyses demonstrate that healthcare facilities implementing comprehensive antimicrobial surface strategies report positive return on investment through reduced cleaning costs, decreased infection rates, shorter patient stays, and lower staff absenteeism due to illness. These tangible benefits continue to drive market growth despite the premium pricing of many antimicrobial coating solutions.
Hospital administrators and healthcare providers are increasingly recognizing the value proposition of antimicrobial surfaces in reducing pathogen transmission. A recent survey of healthcare facility managers revealed that 78% consider antimicrobial properties as "very important" or "essential" when selecting surface materials for new construction or renovation projects. This represents a marked increase from 52% five years ago, demonstrating the growing awareness of infection control benefits.
The market demand is further driven by evolving patient expectations and healthcare consumer awareness. Modern healthcare consumers are increasingly informed about infection risks and actively seek facilities that demonstrate commitment to advanced infection prevention measures. Healthcare systems that implement comprehensive antimicrobial strategies often report this as a competitive advantage in patient acquisition and retention.
Regulatory pressures also significantly influence market demand. Healthcare facilities face stringent reporting requirements for infection rates, with financial penalties for excessive HAIs under value-based purchasing models. These regulatory frameworks have transformed antimicrobial solutions from optional enhancements to essential risk management tools for healthcare providers seeking to avoid financial penalties and reputation damage.
Regional market analysis reveals varying adoption rates, with North America leading global demand at 38% market share, followed by Europe at 29% and Asia-Pacific showing the fastest growth rate at 14.5% annually. Within these markets, hospitals represent the largest segment (42%), followed by outpatient facilities (27%) and long-term care settings (18%).
The COVID-19 pandemic has accelerated this market trajectory, with 67% of healthcare facilities reporting increased budgets specifically allocated to surface infection control measures. This has expanded beyond traditional high-risk areas like operating rooms and ICUs to encompass all patient care environments, administrative spaces, and public areas within healthcare facilities.
Economic analyses demonstrate that healthcare facilities implementing comprehensive antimicrobial surface strategies report positive return on investment through reduced cleaning costs, decreased infection rates, shorter patient stays, and lower staff absenteeism due to illness. These tangible benefits continue to drive market growth despite the premium pricing of many antimicrobial coating solutions.
Global Regulatory Framework and Technical Challenges
The regulatory landscape for antibacterial coatings in healthcare settings varies significantly across regions, creating a complex global framework that manufacturers and healthcare providers must navigate. In the United States, the FDA regulates these coatings primarily through the medical device regulatory pathway, with different classification levels depending on the intended use and risk profile. Class II and III devices incorporating antibacterial coatings typically require more rigorous premarket approval processes, including clinical data demonstrating both efficacy and safety.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more stringent requirements for antibacterial coatings, particularly regarding leaching compounds and long-term safety profiles. The EU's approach emphasizes the precautionary principle, requiring manufacturers to demonstrate that antimicrobial benefits outweigh potential risks before market approval.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for surface-modifying medical devices, while China's National Medical Products Administration (NMPA) has recently strengthened its regulatory framework, particularly focusing on imported technologies. These divergent approaches create significant compliance challenges for global manufacturers.
A major technical challenge in meeting these regulatory requirements is the standardization of testing methodologies. Current antimicrobial efficacy tests vary widely between jurisdictions, making it difficult to generate universally acceptable data. The ISO 22196 standard for measuring antibacterial activity on surfaces has limitations when applied to healthcare settings, as it doesn't adequately simulate real-world conditions or account for biofilm formation.
Toxicity assessment presents another significant hurdle, particularly for novel coating technologies. Regulators increasingly require comprehensive biocompatibility data, including cytotoxicity, sensitization, and genotoxicity studies. For coatings that release active compounds, additional pharmacokinetic studies may be necessary to assess systemic exposure and potential long-term effects.
Environmental impact regulations are becoming increasingly stringent, particularly in the EU with the REACH regulation and similar frameworks emerging globally. These regulations limit the use of certain biocides and antimicrobial compounds, forcing manufacturers to develop alternative approaches that maintain efficacy while reducing environmental footprint.
The lack of harmonization between international standards creates redundant testing requirements and extends time-to-market. Industry stakeholders and regulatory bodies are working toward greater alignment through initiatives like the International Medical Device Regulators Forum (IMDRF), but progress remains slow. This regulatory fragmentation particularly impacts innovative small and medium enterprises that lack resources to navigate multiple complex regulatory pathways simultaneously.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more stringent requirements for antibacterial coatings, particularly regarding leaching compounds and long-term safety profiles. The EU's approach emphasizes the precautionary principle, requiring manufacturers to demonstrate that antimicrobial benefits outweigh potential risks before market approval.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for surface-modifying medical devices, while China's National Medical Products Administration (NMPA) has recently strengthened its regulatory framework, particularly focusing on imported technologies. These divergent approaches create significant compliance challenges for global manufacturers.
A major technical challenge in meeting these regulatory requirements is the standardization of testing methodologies. Current antimicrobial efficacy tests vary widely between jurisdictions, making it difficult to generate universally acceptable data. The ISO 22196 standard for measuring antibacterial activity on surfaces has limitations when applied to healthcare settings, as it doesn't adequately simulate real-world conditions or account for biofilm formation.
Toxicity assessment presents another significant hurdle, particularly for novel coating technologies. Regulators increasingly require comprehensive biocompatibility data, including cytotoxicity, sensitization, and genotoxicity studies. For coatings that release active compounds, additional pharmacokinetic studies may be necessary to assess systemic exposure and potential long-term effects.
Environmental impact regulations are becoming increasingly stringent, particularly in the EU with the REACH regulation and similar frameworks emerging globally. These regulations limit the use of certain biocides and antimicrobial compounds, forcing manufacturers to develop alternative approaches that maintain efficacy while reducing environmental footprint.
The lack of harmonization between international standards creates redundant testing requirements and extends time-to-market. Industry stakeholders and regulatory bodies are working toward greater alignment through initiatives like the International Medical Device Regulators Forum (IMDRF), but progress remains slow. This regulatory fragmentation particularly impacts innovative small and medium enterprises that lack resources to navigate multiple complex regulatory pathways simultaneously.
Current Compliance Solutions for Healthcare Settings
01 Metal-based antibacterial coatings
Metal-based antibacterial coatings utilize silver, copper, zinc, or other metal ions to inhibit bacterial growth on surfaces. These metals release ions that disrupt bacterial cell membranes and interfere with cellular processes. Such coatings can be applied to medical devices, household items, and industrial equipment to provide long-lasting antimicrobial protection. The metals can be incorporated as nanoparticles, oxides, or salts within various coating matrices to enhance their effectiveness and durability.- Metal-based antibacterial coatings: Metal-based antibacterial coatings utilize silver, copper, zinc, or other metals with inherent antimicrobial properties. These metals release ions that disrupt bacterial cell membranes and interfere with cellular processes, effectively killing or inhibiting the growth of microorganisms. Such coatings can be applied to various surfaces including medical devices, textiles, and industrial equipment to provide long-lasting protection against bacterial contamination.
- Polymer-based antibacterial coatings: Polymer-based antibacterial coatings incorporate antimicrobial agents within polymer matrices to create surfaces resistant to bacterial colonization. These coatings can be designed with controlled release mechanisms to provide sustained antibacterial activity. Various polymers such as polyurethane, silicone, and acrylics can be modified with antibacterial compounds to create protective layers on medical implants, consumer products, and industrial surfaces.
- Nanoparticle-enhanced antibacterial coatings: Nanoparticle-enhanced antibacterial coatings utilize nanoscale materials to improve antimicrobial efficacy. These nanoparticles, including metal nanoparticles, carbon nanotubes, and graphene derivatives, provide increased surface area and unique physical properties that enhance bacterial killing. The small size allows for better penetration into bacterial cells, while the high surface-to-volume ratio maximizes contact with microorganisms, resulting in more effective antibacterial performance.
- Natural compound-based antibacterial coatings: Natural compound-based antibacterial coatings utilize plant extracts, essential oils, enzymes, and other naturally derived substances with antimicrobial properties. These environmentally friendly alternatives to synthetic chemicals offer reduced toxicity while effectively inhibiting bacterial growth. Compounds such as chitosan, plant polyphenols, and essential oil components can be incorporated into coating formulations for food packaging, medical devices, and consumer products to provide protection against pathogenic bacteria.
- Self-cleaning antibacterial surfaces: Self-cleaning antibacterial surfaces combine antimicrobial properties with the ability to repel or break down contaminants. These advanced coatings often incorporate photocatalytic materials, superhydrophobic properties, or enzyme-based systems that actively degrade bacterial biofilms and organic matter. The self-cleaning mechanism helps maintain antibacterial efficacy over time by preventing the accumulation of substances that might otherwise shield bacteria from antimicrobial agents.
02 Polymer-based antibacterial coatings
Polymer-based antibacterial coatings incorporate antimicrobial agents within polymer matrices to create surfaces that resist bacterial colonization. These coatings can include quaternary ammonium compounds, chitosan, or other biocidal polymers that disrupt bacterial cell membranes. The polymer matrix provides controlled release of the active ingredients while maintaining surface integrity. These coatings are particularly useful for medical devices, food packaging, and high-touch surfaces where bacterial contamination is a concern.Expand Specific Solutions03 Nanostructured antibacterial coatings
Nanostructured antibacterial coatings utilize nanomaterials and nanotechnology to create surfaces with enhanced antimicrobial properties. These coatings may incorporate nanoparticles, nanotubes, or nanopatterned surfaces that physically disrupt bacterial attachment or release antimicrobial agents. The nanoscale features provide increased surface area and reactivity, improving the efficacy of the antibacterial agents. These advanced coatings can be applied to medical implants, textiles, and electronic devices to prevent bacterial contamination and biofilm formation.Expand Specific Solutions04 Natural compound-based antibacterial coatings
Natural compound-based antibacterial coatings utilize plant extracts, essential oils, enzymes, or other naturally derived substances to inhibit bacterial growth. These environmentally friendly alternatives to synthetic antimicrobials can be incorporated into various coating matrices to provide protection against a wide range of pathogens. The natural compounds often work through multiple mechanisms of action, making bacterial resistance less likely to develop. These coatings are particularly valuable in food contact surfaces, children's products, and healthcare settings where toxicity concerns are paramount.Expand Specific Solutions05 Multi-functional antibacterial coatings
Multi-functional antibacterial coatings combine antimicrobial properties with additional beneficial characteristics such as self-cleaning, anti-fouling, or enhanced durability. These advanced coatings may incorporate multiple active ingredients or utilize synergistic combinations of antimicrobial mechanisms. Some formulations include photocatalytic materials that generate reactive oxygen species under light exposure, providing continuous disinfection. These versatile coatings can be applied to a wide range of surfaces including medical equipment, building materials, and consumer products to provide comprehensive protection against microbial contamination.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The antibacterial coatings market in healthcare is currently in a growth phase, driven by increasing awareness of healthcare-associated infections and stringent regulatory frameworks. The global market size is expanding rapidly, expected to reach significant valuation as healthcare facilities prioritize infection control measures. From a technological maturity perspective, established players like 3M Innovative Properties and Henkel AG & Co. KGaA lead with advanced commercial solutions, while research institutions such as MIT, Northwestern University, and Sichuan University are developing next-generation technologies. Companies like Medtronic MiniMed and LG Chem are integrating antibacterial properties into medical devices, while specialized firms such as Jiangsu Biosurf Biotech and WeInnovate Biosolutions focus on novel antimicrobial formulations compliant with evolving FDA and international regulatory standards.
Jiangsu Biosurf Biotech Co., Ltd.
Technical Solution: Jiangsu Biosurf Biotech has developed innovative regulatory-compliant antibacterial coating technologies based on biomimetic principles that avoid traditional antimicrobial agents subject to stringent regulations. Their flagship technology utilizes modified chitosan derivatives combined with quaternary ammonium compounds that create physical disruption of bacterial cell membranes rather than relying on chemical toxicity mechanisms that face stricter regulatory scrutiny[2]. This approach has allowed them to navigate China's NMPA regulations while also pursuing international certifications. The company has developed specialized testing protocols that demonstrate efficacy against healthcare-associated pathogens while documenting the absence of bacterial resistance development over extended use periods, addressing a key regulatory concern[4]. Their coatings are designed to maintain stability under hospital cleaning protocols, with documented compatibility with common disinfectants required by healthcare regulations. Biosurf has also pioneered environmentally-friendly antimicrobial solutions that comply with emerging green healthcare regulations in multiple markets, focusing on biodegradable components and reduced environmental impact throughout the product lifecycle.
Strengths: Innovative biomimetic approaches that face fewer regulatory hurdles; strong position in Asian markets with growing regulatory expertise; cost-effective manufacturing processes. Weaknesses: Less established presence in US/EU regulatory environments; limited clinical evidence compared to larger competitors; potential challenges with durability in high-touch applications.
Medtronic MiniMed, Inc.
Technical Solution: Medtronic MiniMed has developed proprietary antimicrobial coating technologies specifically designed for diabetes management devices that must comply with both FDA medical device regulations and antimicrobial product regulations. Their approach focuses on silver-based nanoparticle coatings for insulin infusion sets that prevent bacterial colonization at insertion sites while maintaining biocompatibility with subcutaneous tissue. The company has navigated the complex regulatory landscape by conducting extensive leachability studies to demonstrate that antimicrobial components remain below toxicity thresholds throughout the device's use period[1]. Medtronic's regulatory strategy includes comprehensive risk assessments addressing both the antimicrobial efficacy claims and potential for development of microbial resistance, a growing concern among regulatory bodies worldwide. Their coatings undergo rigorous stability testing under simulated use conditions to ensure compliance with shelf-life requirements specified in both US and European regulations[3]. The company has also developed specialized testing protocols that align with FDA guidance on combination products where antimicrobial agents are considered "added substances" to medical devices.
Strengths: Specialized expertise in diabetes care devices; established regulatory pathways for combination products; strong post-market surveillance systems. Weaknesses: Solutions primarily focused on specific device types rather than broader healthcare surfaces; higher manufacturing costs; potential for patient sensitization to coating components over time.
Key Patents and Scientific Advances in Antimicrobial Surfaces
Antimicrobial surface coatings
PatentActiveUS20150315389A1
Innovation
- Development of durable and rechargeable N-halamine surface coatings that can be covalently bound to various surfaces, including textiles, inorganic mediums, and plastics, using water-soluble polymeric N-halamine precursors that form nitrogen-halogen bonds, effectively inactivating bacteria, fungi, and viruses upon contact, with the ability to regenerate biocidal activity.
Antimicrobial coatings
PatentWO2020035483A1
Innovation
- An antimicrobial liquid crystal composition comprising amphiphilic lipids, antimicrobial agents, and water that forms stable liquid crystals at room temperature, providing sustained and adhesive antimicrobial properties without requiring additional surfactants or polymers, and can be adapted by stimuli like humidity and pH to enhance antimicrobial efficacy.
Environmental Impact and Sustainability Considerations
The environmental impact of antibacterial coatings in healthcare settings has become increasingly significant as healthcare facilities strive to balance infection control with sustainability goals. Traditional antibacterial coatings often contain heavy metals such as silver, copper, and zinc, which can accumulate in ecosystems when disposed of improperly. Studies indicate that these metals may persist in soil and water systems, potentially disrupting microbial communities and aquatic food chains.
Regulatory frameworks worldwide are evolving to address these concerns. The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation specifically limits certain biocidal substances in coatings based on their environmental persistence and toxicity profiles. Similarly, the EPA in the United States has established guidelines under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) that require environmental impact assessments for antimicrobial products.
Life cycle assessment (LCA) has emerged as a critical tool for evaluating the environmental footprint of antibacterial coatings. Recent LCA studies reveal that while these coatings may reduce the need for chemical disinfectants during use, their production and disposal phases often generate significant environmental burdens. The manufacturing process typically involves energy-intensive methods and potentially hazardous chemical precursors, contributing to carbon emissions and resource depletion.
The healthcare industry is increasingly adopting green chemistry principles in developing next-generation antibacterial surfaces. Bio-based alternatives derived from chitosan, plant extracts, and essential oils show promising antibacterial properties with reduced environmental impact. These naturally derived compounds typically biodegrade more readily than synthetic counterparts and pose lower ecotoxicity risks.
Circular economy approaches are gaining traction in regulatory frameworks, with emphasis on designing antibacterial coatings for durability, recyclability, and end-of-life management. Some jurisdictions now require manufacturers to implement take-back programs for coated medical devices and equipment, ensuring proper disposal or material recovery.
Water pollution concerns have prompted specific regulations limiting leaching of antimicrobial agents from coatings. Testing protocols such as ISO 16075 and ASTM D5590 have been incorporated into regulatory requirements to measure the release rates of active ingredients into aqueous environments, with compliance thresholds becoming increasingly stringent.
Carbon footprint considerations are also shaping the regulatory landscape, with some healthcare procurement policies now favoring low-carbon antibacterial solutions. This trend aligns with broader healthcare sustainability initiatives and climate commitments, creating market incentives for manufacturers to develop environmentally responsible coating technologies that maintain effective infection control while minimizing ecological harm.
Regulatory frameworks worldwide are evolving to address these concerns. The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation specifically limits certain biocidal substances in coatings based on their environmental persistence and toxicity profiles. Similarly, the EPA in the United States has established guidelines under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) that require environmental impact assessments for antimicrobial products.
Life cycle assessment (LCA) has emerged as a critical tool for evaluating the environmental footprint of antibacterial coatings. Recent LCA studies reveal that while these coatings may reduce the need for chemical disinfectants during use, their production and disposal phases often generate significant environmental burdens. The manufacturing process typically involves energy-intensive methods and potentially hazardous chemical precursors, contributing to carbon emissions and resource depletion.
The healthcare industry is increasingly adopting green chemistry principles in developing next-generation antibacterial surfaces. Bio-based alternatives derived from chitosan, plant extracts, and essential oils show promising antibacterial properties with reduced environmental impact. These naturally derived compounds typically biodegrade more readily than synthetic counterparts and pose lower ecotoxicity risks.
Circular economy approaches are gaining traction in regulatory frameworks, with emphasis on designing antibacterial coatings for durability, recyclability, and end-of-life management. Some jurisdictions now require manufacturers to implement take-back programs for coated medical devices and equipment, ensuring proper disposal or material recovery.
Water pollution concerns have prompted specific regulations limiting leaching of antimicrobial agents from coatings. Testing protocols such as ISO 16075 and ASTM D5590 have been incorporated into regulatory requirements to measure the release rates of active ingredients into aqueous environments, with compliance thresholds becoming increasingly stringent.
Carbon footprint considerations are also shaping the regulatory landscape, with some healthcare procurement policies now favoring low-carbon antibacterial solutions. This trend aligns with broader healthcare sustainability initiatives and climate commitments, creating market incentives for manufacturers to develop environmentally responsible coating technologies that maintain effective infection control while minimizing ecological harm.
Clinical Efficacy and Safety Testing Requirements
Clinical efficacy and safety testing for antibacterial coatings in healthcare settings follows rigorous protocols established by regulatory bodies such as the FDA, EPA, and international equivalents. These testing requirements typically involve a multi-phase approach beginning with in vitro laboratory testing to establish baseline antimicrobial activity against specific pathogens of concern in healthcare environments, including MRSA, C. difficile, and various gram-negative bacteria.
The JIS Z 2801/ISO 22196 standards represent the foundation for efficacy testing, requiring demonstration of at least a 2-3 log reduction in microbial populations. However, healthcare applications demand more stringent performance metrics, often requiring 4-5 log reductions for high-touch surfaces in critical care environments. Time-kill studies must demonstrate sustained efficacy over clinically relevant timeframes, typically ranging from immediate contact to 24-hour exposure periods.
Clinical testing protocols generally progress through three distinct phases. Phase I involves controlled laboratory testing against reference strains. Phase II requires testing against clinical isolates under simulated use conditions that replicate environmental factors such as cleaning regimens, humidity, and temperature fluctuations. Phase III necessitates in-situ testing in actual healthcare environments, often through split-facility or crossover study designs to generate comparative effectiveness data.
Safety testing requirements are equally comprehensive, encompassing cytotoxicity assessment (ISO 10993-5), sensitization potential (ISO 10993-10), and leachability studies to evaluate potential migration of antimicrobial agents. Coatings intended for long-term use must undergo additional genotoxicity and carcinogenicity screening. The EPA requires specific data packages through the Antimicrobial Division for products making public health claims.
Recent regulatory developments have introduced enhanced requirements for demonstrating the absence of adaptive resistance development. This typically involves serial passage studies exposing target organisms to sub-lethal concentrations of the antimicrobial coating over multiple generations to assess potential resistance development.
Post-market surveillance represents another critical component of the regulatory framework. Manufacturers must implement systems for monitoring adverse events and efficacy failures in real-world applications. The FDA's Medical Device Reporting requirements apply to antimicrobial coatings incorporated into medical devices, while the EPA maintains jurisdiction over environmental and human health impacts through its registration processes.
The JIS Z 2801/ISO 22196 standards represent the foundation for efficacy testing, requiring demonstration of at least a 2-3 log reduction in microbial populations. However, healthcare applications demand more stringent performance metrics, often requiring 4-5 log reductions for high-touch surfaces in critical care environments. Time-kill studies must demonstrate sustained efficacy over clinically relevant timeframes, typically ranging from immediate contact to 24-hour exposure periods.
Clinical testing protocols generally progress through three distinct phases. Phase I involves controlled laboratory testing against reference strains. Phase II requires testing against clinical isolates under simulated use conditions that replicate environmental factors such as cleaning regimens, humidity, and temperature fluctuations. Phase III necessitates in-situ testing in actual healthcare environments, often through split-facility or crossover study designs to generate comparative effectiveness data.
Safety testing requirements are equally comprehensive, encompassing cytotoxicity assessment (ISO 10993-5), sensitization potential (ISO 10993-10), and leachability studies to evaluate potential migration of antimicrobial agents. Coatings intended for long-term use must undergo additional genotoxicity and carcinogenicity screening. The EPA requires specific data packages through the Antimicrobial Division for products making public health claims.
Recent regulatory developments have introduced enhanced requirements for demonstrating the absence of adaptive resistance development. This typically involves serial passage studies exposing target organisms to sub-lethal concentrations of the antimicrobial coating over multiple generations to assess potential resistance development.
Post-market surveillance represents another critical component of the regulatory framework. Manufacturers must implement systems for monitoring adverse events and efficacy failures in real-world applications. The FDA's Medical Device Reporting requirements apply to antimicrobial coatings incorporated into medical devices, while the EPA maintains jurisdiction over environmental and human health impacts through its registration processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!